PRINCIPLES OF FOOD FREEZING
Development of a Frozen Food Industry
Freezing temperatures, once feared by mankind, have been turned to his great advantage by his inquiry into the phenomena. While ice-salt systems were used to freeze foods in the mid 1800's, and patents for freezing fish, for example, were granted in 1861 to Enoch Piper in Maine, and even earlier to H. Benjamin in England in 1842 the invention of mechanical refrigeration in the late 1800's provided the base for subsequent commercial exploitation of the process. Frozen foods have become important items of commerce (90 per cent of Iceland's export trade is frozen fish) and important in food preparation for dinner tables (Figs. 4.1 8s 4.2).
Clarence Birdseye fathered this revolution as a technologist by developing quick freezing processes and equipment, and successfully promoting consumer units of frozen foods. He overcame tremendous obstacles. In the 1920's there "were few mechanical refrigerators in homes in the United States.
In the 1930's, as facilities for food freezing and retail distribution developed across the United States, frozen foods began to find their place in commerce. Yet, it was not until 1940 that they became important competitors of other consumer-type preserved foods. While Clarence Birdseye was a prime mover industrially, the frozen food industry had support in the scientific aspects of the development by men such as Dr. Donald K. Tressler, at Cornell, and Dr. C.R. Fellers at the then Massachusetts State College.
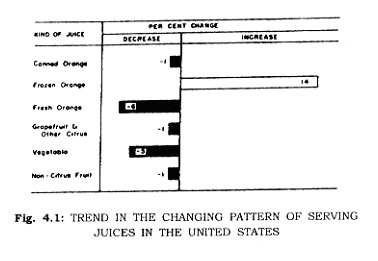
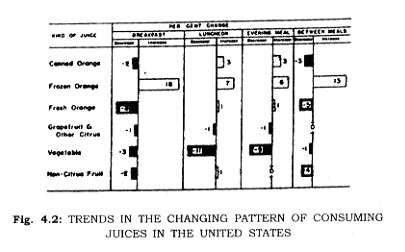
The present day finds competition between all methods of food preservation, and the competition is being resolved by consumers (Fig. 4.2). Those foods best preserved by freezing are largely frozen. Those foods highly acceptable as canned products continue as highly successful consumer goods. The economic struggle for survival between fresh commodities, canned foods, and frozen foods in a free market evidences itself in better foods at lower prices for consumers.
The Freezing Point of Foods
Living cells contain much water, often two-thirds or mgre of their weight. In this medium there are organic and inorganic substances, including salts and sugars and acids in aqueous solutions, and more complex organic molecules such as proteins which are colloidal suspension. To some extent gases are also dissolved in the watery solution.
The physical, chemical, and biological changes occurring during the freezing and subsequent thawing of foods are complex and not completely understood. Nevertheless it is useful to study the nature of these changes which have been recognized in order to design a successful freezing process for a food.
The freezing point of a liquid is that temperature at which the liquid is in equilibrium with the solid. A solution with a vapour pressure lower than that of a pure solvent will not be in equilibrium with the solid solvent is at normal freezing point. The system must be cooled to that temperature at which the solution and the solid solvent have the same vapour pressure. The freezing point of a solution is lower than that of a pure solvent. The freezing point of food is lower than that of pure water.
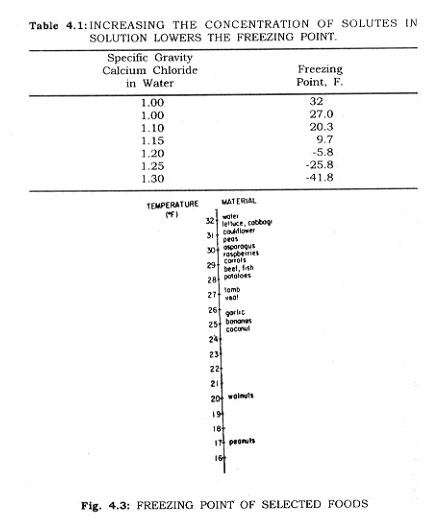
When a liquid evaporates the escaping molecules exert a pressure known as the vapour pressure. The total pressure of a system will be equal to the sum of the partial pressures of the system. The addition of a non-volatile solute (sugar) to water lowers the vapour pressure of the water solution of sugar, and the freezing point of the water solution will be lower than that of pure water (Table 4.1).
Because of the high content of water in most foods, most of them freeze solidly at temperatures between 32° and 25°F (Fig. 4.3). The temperature of the food undergoing freezing remains relatively constant until the food is mostly frozen, after which time the temperature approaches that of the freezing medium. Quick freezing has been defined, by those who adhere to rapid crystallization theory, as that process where the temperature of the food passes through the zone of maximum ice crystal formation (32° to 25°F.) in 30 minutes or less. The basic principle of all rapid freezing methods is the speedy removal of heat from food. These methods include freezing in cold air blasts, by direct immersion of the food in a cooling medium, by contact with refrigerated* plates irf a freezing chamber, and by freezing with liquid air, nitrogen, or carbon dioxide. Freezing in still air is the poorest method of all. By circulating cold air, the freezing rate is greatly accelerated, as will be explained.
Per Cent Water Frozen vs. Temperature of Food and Its Quality
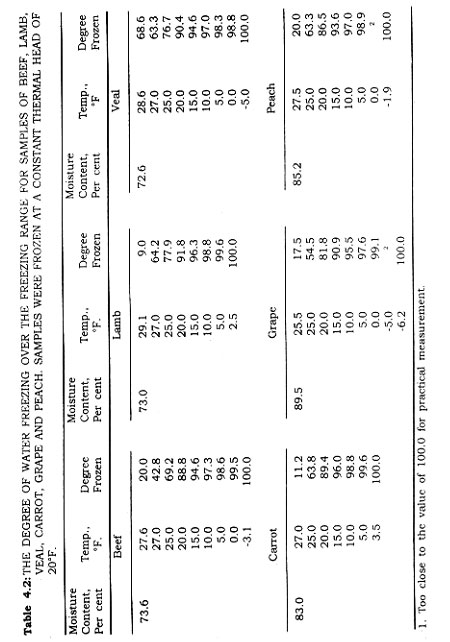
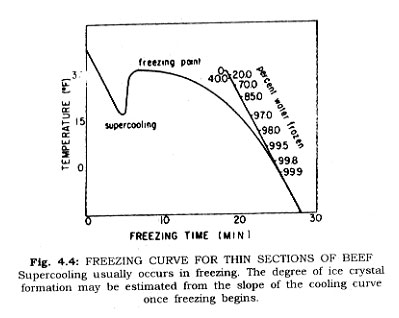
It is a well established fact that foods freeze over a wide range of temperatures, although their freezing points are identifiable. This is demonstrated in Fig. 4.3. Careful evaluation of the freezing curve for a food, beef for example, under controlled conditions, demonstrates first that supercooling occurs, and that this is characteristic for products. With a thin section of food tissue it can be shown that following supercooling the temperature of the cooled section rises to the actual freezing point when the change in phase occurs. This change in phase continues, providing a temperature differential is maintained, until the free water becomes ice.
It is a fact that the water in foods exists in two (or more) states. The generally used terms are "bound" water and "free" water. Some accept the definition of bound water as that which does not freeze at -5°F. Free water on the other hand exhibits the physical and chemical properties of liquid water, and freezes according to its condition of solution. In fish flesh, researchers have found that even at -30°F. there remains some non-crystalline water. True, the amount is infinitesimal. Fig. 4.4 shows the per cent water frozen in a sample of food at different temperatures. A satisfactory definition of bound water is difficult to make, but good evidence exists for its justification. Bound water exists in proportion of the free water content rather than the solid material of the system. There may be a shift due to freezing in the amount of bound water (Table 4.2).
Reducing the amount of free water in a food can therefore be expected to improve the quality of the frozen food. The more complete the change from free water to a major stable state, the better is the retention of quality in the frozen food.
Flavour changes, color changes, nutrient losses, and texture losses occur relatively rapidly above 15°F. (as compared to 0°F. or lower). The lower the temperature, the slower the rate of loss of ascorbid acid. Further, some products deteriorate more rapidly under fluctuating temperatures.
Fresh fish flesh is gelatinous, and if its temperature is lowered rapidly to -40°F., when thawed the flesh loses little tissue fluid. On the other hand, if slowly frozen the flesh may, when thawed, lose much as "drip." There is a point of electrolyte concentration attained in the tissue which may cause irreversible changes in colloidal structure.
Dehydro-freezing is a combination process of dehydration followed by freezing. As indicated above, the processes which reduce free water in foods do not damage the quality of the frozen product, providing the removal of water itself does not cause deleterious changes in the food substrate.
Size of Ice Crystals Formed
Under standard conditions the temperature of water must fall"below 32°F. before ice crystals form. When forming, the temperature of the ice-water slurry returns to 32°F. If ice crystals are allowed to form slowly, relatively large crystals are produced. If the water is made to freeze rapidly, due to rapid heat removal from the system, the ice formed will have a fine texture. Looking at the ice formed, in the first instance there are large, sharp needle-like structures, and in the latter instance, there are many more and smaller ice crystals. If a fine textured ice is partially allowed to melt, then refrozen, and the process repeated several times, the ice crystals will change from small to large.
Ice cream made simply by placing it in an ice cube tray of a small home refrigerator will develop large ice crystals, and the ice cream will have a "coarse" texture, unlike the usual rapidly frozen commercial product, which has a velvet-smooth ice crystal system. This phenomenon is equally demonstrated with strawberries. Fruit placed in such a tray and frozen slowly will be lacking characteristic texture when thawed. Piercing ice crystals form, puncturing tissue cells. When thawed the cells spew their contents; the berry is flabby and loses its form.
According to the crystal damage theory, ice crystal growth impairs food quality generally. Slow freezing permits ice crystal growth. The cells of meat, poultry, fish, shellfish, fruits, and vegetables all contain jelly-like protoplasm. To fix the original jelly-like mass, the rate of freezing must be such that minute crystals from uniformly, throughout the tissues. If such quick frozen tissue is thawed immediately the water is reabsorbed within the tissues as the ice crystals melt. If the product is frozen slowly, or fluctuating conditions of temperature during storage permit ice crystal growth, cells become punctured and the thawed tissues can not return to their original jelly-like state. Some of the fluid resulting from the thawing does not become reabsorbed and appears as free liquid.
However, as noted above, ice crystal growth is but one of the factors influencing the quality of frozen foods. As freezing progresses through a substrate, there is an increasing concentration of electrolytes which may cause irreversible changes in colloidal structures. It is not uncommon to observe coagulated protein in thawed milk, for example, or to find curdled, thawed cream sauces. Damage to the structure of tissues can result from ice crystal growth. Both phenomena occur simultaneously; each is important.
Volume Changes During Freezing
In a freezing environment an unprotected bottle of milk freezes, and, if it does, it usually pushes the cap from the bottle. The milk expands on freezing; the volume of milk increases. The cap sits on top of a protruding cylinder of frozen milk. If the milk bottle has a solidly held cap of metal, one that would resist being pushed from the bottle, then it would burst. The liquid is not compressible, and neither is the ice. As the volume increases within the bottle during freezing, either the bottle bursts, or the cap is forced free.
When freezing food in a rigid container, opportunity for such expansion must be considered.
However not all food products expand during freezing. Strawberry jam does not increase in volume when freeze. The frozen sugary solution occupies the same height in the jar frozen as unfrozen-and it may even occupy less. Water alone can be expected to increase about ten per cent in volume on freezing. With high sugar concentrations in water, the expansion may be nil and actually a decrease in volume can occur. Strawberries packed in sugar as ordinarily frozen will expand slightly on freezing.
Refrigeration Requirements in Freezing Foods
Hot and cold are relative concepts, requiring a reference point. The term ice carries information, and in its common usage, refers to the solid state of water molecules. Water freezes below 32°F. To keep water molecules in the solid state, it is necessary to have them in an environment which permits the solid state to exist.
In colder climates during the winter this presents little difficulty. In warmer climates, it is necessary to create an artificial environment in order to obtain, and maintain, water in a solid state. First a condition must be established such that heat can be removed from liquid water, and a change in state permitted to occur. Next, the frozen material must be protected (insulated) to prevent it from acquiring heat, raising the temperature, and thawing the ice formed. There are two distinct problem areas; the problem of bringing a sample to a frozen condition, and the problem of maintaining the frozen material in a suitable solid state. In order to establish the refrigeration requirements to achieve this desired state, it is necessary to consider both aspects of the problem.
Establishing the Refrigeration Requirements to Freeze Food
The temperature at which a food will freeze, under standard conditions, is dependent upon the concentration of solutes in the water phase. A temperature of 28°F. is usable as the average freezing point of foods in general.
To freeze a food, it is first necessary to bring the temperature of the mass down to the freezing point. The work required to accomplish this is shown in the following equations:
H1= (SL) (W) (Ti - Tf)
where
H1 = B.t.u. required to lower the temperature of the food from the initial temperature (Tt) to the temperature at which the food freezes (Tf).
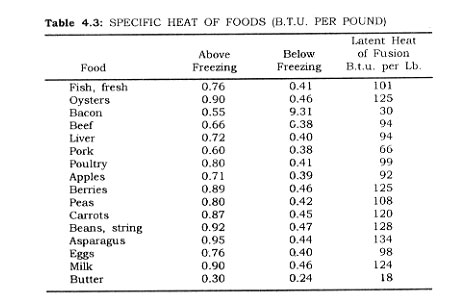
SL = the specific heat of the food above the freezing point of the food (Table 24).
W = the weight of the food mass in pounds.
Example: What is the B.t.u. requirement to lower the temperature of 1,000 Ibs. of peas from 70°F. to the freezing point of peas (30°F.)?
H1 = (0.8) (1.000) (70-30) = 32,000 B.t.u.
To freeze a food, it is necessary to remove the heat of fusion, once the food mass has been brought to the freezing point. The work required to accomplish this is shown as follows:
H2 = (Hf) (W)
where
H2 = B.t.u. required to change liquid food at freezing point, to solid state, at freezing point.
H1 = heat of fusion, in B.t.u. per pound (Table 4.3).
W = weight of food in pounds.
Example: What is the B.t.u. requirement to-tfreeze 1?000 Ibs. of peas at their freezing point?
H2 = (108) (1,000) = 108,000 B.t.u.
Once food is frozen, the temperature of the frozen mass must be lowered to the frozen storage temperature. Commercial frozen storage temperature in the United States is usually 0°F. although lower temperatures are often desirable. Work is required to bring the temperature of the frozen mass, at its freezing point, to the temperature of the storage chamber, and it can be calculated as follows:
H3 = (S8) (W) (Tf - T8)
Where
H3 = B.t.u. required to lower the temperature of the mass (W) from the freezing point (Tf) to the storage temperature (T8).
S8 = the specific heat of the frozen material (Table 4.3).
Example: What is the B.t.u. requirement to bring the temperature of 1,000 Ibs. of frozen peas at 30°F. to the storage temperature of 0°F.?
H3 = (0.42) (1,000) (30 - 0) = 12,600 B.t.u.
The refrigeration requirement to freeze food and bring its temperature to frozen storage temperatures is a sum of the values Hp H2 and H3, and is reported usually in terms of the B.t.u. load. In the above example, the B.t.u. requirement for 1,000 Ibs. of peas at a temperature of 70°F. to be frozen and brought to a storage temperature of 0°F. is as follows:
Hf8 = H1 + H2 + H3 where
Hf8 = is the B.t.u. load requirement to freeze the food mass and lower the temperature of the mass to frozen storage temperature.
Hf8 = 32,000 + 108,000 + 12,600 = 152,600 B.t.u.
The refrigeration requirement (Rf) solely for the frozen food mass is generally reported in terms of tons of refrigeration needed. The value is obtained by changing the B.t.u. load (HB) into equivalent tons of refrigeration. This is accomplished by the following:
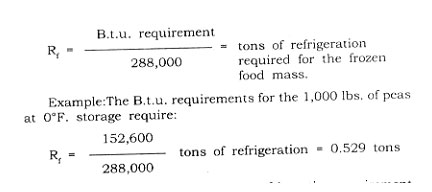
The next step is to establish the refrigeration requirement for the chamber to hold the frozen food in a solid state at a suitable given temperature. The purpose of this discussion it is assumed that the temperature of the frozen food chamber is lower than its environment.
In order to maintain a frozen mass of foods at a desired temperature, it is necessary to insulate the food and create an artificial environment. A suitable chamber must be designed in order to create a desired micro-environment. "In the ca!fee of a frozen food storage chamber, it is necessary to prevent the transfer of heat from surrounding to the frozen food. There are several considerations. First, there is no perfect insulation system; there will be heat losses through the chamber walls. Second, to be functional, there must be an access to the frozen chamber; there will be heat losses due to opening and closing the frozen food storage chamber. Third, there will be numerous other losses, including heat given to the chamber by electric lights or running electric motors in the chamber, and, there is heat given off by persons working in the micro-environment. These can be estimated as follows:
The refrigeration requirements to maintain an empty frozen food storage chamber at a desired storage temperature may be calculated.
There is work required in maintaining a chamber at a given temperature. The work required in terms of B.t.u. requirements is dependent upon the temperature at which the chamber is to be maintained, the temperature of the outside air, the surface area of the storage chamber, the amount and kind of insulation material applied to the chamber.
The B.t.u. load to maintain a chamber at a given temperature for 24 hours can be calculated from the following equation:
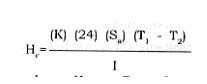
where Hc = B.t.u. losses per 24 hours in the frozen storage chamber. K is the thermal conductivity of the insulation material, Sa is the surface area of the outer wall of the storage chamber. Tt and T2 the temperature outside and inside the chamber, respectively. I is the thickness of the insulation material in inches.
The thermal conductivity (K) and density of commonly used insulating material are shown in Table 4.4.
Example: What are the daily heat losses (Hc) for 0°F. room of outside dimensions 20 ft. high, 20 ft. wide and 40 ft. long, insulated with four inches of corkboard, with an outside temperature of 80°F? How does the corkboard compare with four-inch thick oak wood walls?
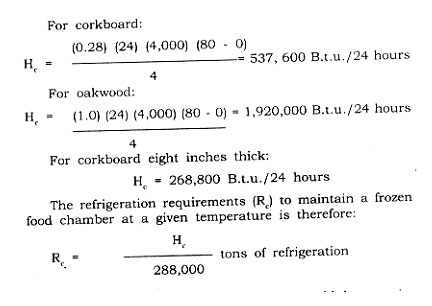
The four-inch corkboard example above would then require 1.86 tons, the four-inch oak wood unit would require 6.67 tons, and the eight-inch corkboard inch 0.93 tons of refrigeration.
Miscellaneous Heat Load in Maintaining and Operating a Frozen Food Chamber.
In addition to the work required in terms of B.t.u. for both the food mass and the storage chamber, there are heat loads in lighting the chamber, in running motors, in having people operating within the chamber, and the opening and closing of the chamber doors. The latter consideration is one involving the changes of air due to entrances and exits from the chamber. The following values are useful in establishing the miscellaneous heat loads :
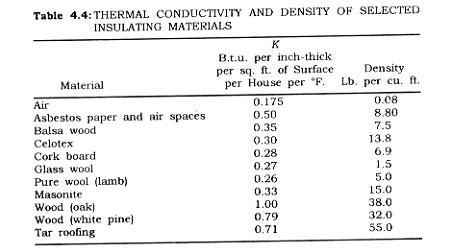
Electric motors = 3,000 B.t.u. per hour per h.p.
Working man (within chamber) = 750 B.t.u. per man hour
Average air changes per 24 hours is dependent upon the size Of the chamber and its use, which may be estimated from Table 4.5.
The average B.t.u. losses due to air changes within a chamber are related to the temperature and relative humidity of the exchanged air.
The heat load (He) contributed by electric light bulbs and running motors can be calculated from the following:
He =3.42 (total lighting watts) (hours burning) + [3,000 (total motor h.p.) (hours running)]
The heat load (He) contributed by these miscellaneous sources will be the sum of the individual contributions.
Example: What is the heat load contributed by five 100-watt electric light bulbs and a 5 h.p. motor, each operating five, hours daily in the frozen storage chamber?
He = 3.42 (500) (5) + 3,000 (5) (5) = 83,550 B.t.u./24 hours
The heat load contributed by men (HJ working within the storage chamber can be calculated from the following:
Hm = 750 (No. of man hours in chamber)
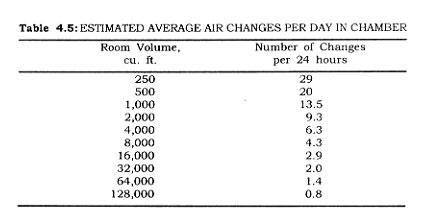
Example: What is the heat load contributed by two men working three hours each in a frozen food chamber?
Hm = 750 (2) (3) = 4,500 B.t.u. per 24 hours
The heat load contributed by opening and closing the chamber is dependent upon the number of times a day the chamber is opened, how long it is left open, the temperature and humidity of the air inside the chamber, and the temperature and humidity of the outside air. One method developed gives the number of air changes estimated during a 24-hour period, shown in Table 4.5. A 1,000 cu. ft. chamber can be estimated to change air about 13 times a day if the chamber is used. Obviously this is a generalization. The B.t.u. per cu. ft. of air removed from the chamber and replaced by warmer outside air can be estimated satisfactorily nevertheless. It will be seen that a 0°F. storage area operating in an 80°F. environment, with air containing 60 per cent relative humidity, will contribute a heat load of 2.9 B.t.u. per cu. ft. of air exchanged. Having at least an estimate of the number of air changes one might expect, and the B.t.u. load per cu. ft. of air changed, it is possible to anticipate the heat load and plan for this operating condition.
PRINCIPLES OF FOOD PRESERVATION BY DRYING
Drying a Natural Process
Drying is one of man's oldest methods of food preservation. It is a process copied from nature; we have improved certain features of the operation. Drying is the most widely used method of food preservation.
All the cereal grains are preserved by drying, and the natural process is so efficient it hardly requires added effort by man. However, there have been periods in history when climatic factors were such that grain failed to dry properly in the fields. In these instances, man attempted to assist the natural action by supplying heat to the grains which otherwise would decompose. Grains, legumes, nuts, and certain fruits mature on the plants, and dry in the warm wind. More fruits are preserved by drying than by any other method of food preservation. The natural sun drying of foods yield highly concentrated materials of enduring quality, yet a highly complex civilization can not be so dependent upon the elements-they are unpredictable. Sun drying remains the greatest food preservation action.
Dehydration-Artificial Drying
The use of heat from a fire of dry foods was discovered independently by many men in the New and Old Worlds. Ancient man dried foods in his shelters; pre-Columbus American Indians used the heat from fire to dry foods. But, it was not until about 1795 that a hot air dehydration room was invented. The team of Masson and Challet in France developed a vegetable dehydrator which consisted of a hot air (105°F) flow over thin slices of vegetables. It is worth nothing that both canning and dehydration came into being at approximately the same time, nearly a century and half ago.
Evaporation and desiccation are terms which perhaps note the same action. The term dehydration has taken the meaning artificial drying; dehydration has assumed meaning in the food industry as that process of artificial drying.
Dehydration vs. Sun Drying
Dehydration implies control over climatic conditions within a chamber, or micro-environment control. Sun drying is at the mercy of the elements. Dried foods from a dehydration unit can have better quality than sun dried counterparts. Less land is required for the drying activity. Sun drying for fruit requires approximately one acre of drying surface per 20 acres of crop land.
Sanitary conditions are controllable within a dehydration plant, whereas in open fields contamination from dust, insects, birds, and rodents are major problem.
Dehydration obviously is a more expensive process than sun drying, yet the dried foods may have more monetary value from dehydration due to improved quality. The yield of dried fruit from a dehydrator is higher inasmuch as sugar is lost due to continued respiration of tissues during sun drying, and also due to fermentation.
The color of sun dried fruit may be superior to dehydrated fruit under optimum conditions of operation of both. Color development in certain immature fruits continues slowly during sun drying. This does not occur during dehydration.
In cooking quality dehydrated foods are usually superior to sun dried counterparts. However, sun dried animal flesh and fish can be highly acceptable.
On the basis of cost sun drying has advantages, but on the basis of time to dry, and quality, dehydration has merits. Furthermore sun drying can not be practiced widely due to unfavourable weather conditions in many areas where man lives and agriculture is rewarding.
Why Dried Foods
Dried and dehydrated foods are more concentrated than any other preserved form of foodstuffs. They are less costly to produce; there is a minimum of labor required, processing equipment is limited, dried food storage requirements are at a minimum, and distribution costs are reduced (one car load of dried, compressed food may equal ten car loads of the fresh commodity, see Tables 5.1 and 5.2).
Dehydration Permits Food Preservation
There are chemical and biological forces acting upon the food supply man desires. Man controls the chemical forces in dehydrated food by packaging and certain chemical additives. The biological forces are controlled by reducing the free water content and by heating. To be a suitable substrate to support growth of micro-organisms, a food must have free water, available for the micro-organisms. By reducing the free water content, thereby increasing osmotic pressures, microbial growth can be controlled.
Air-The Drying Medium
Foodstuffs may be dried in air, superheated steam, in vacuum, in inert gas, and by the direct application of heat. Air is generally used as the drying medium because it is plentiful, convenient, and overheating of the food can be controlled. Air is used to conduct heat to the food being dried, and to carry liberated moisture vapor from the food. No elaborate moisture recovering system is required with air, as is needed with other gases. Drying can be accomplished gradually, and tendencies to scorch and discolor are within control.
Function of Air in Drying-Air conveys heat to the food, causing water to vaporize, and is the vehicle to transport the liberated moisture vapor from the dehydrating food.
Volume of air Required in Drying-More air is required to conduct heat to the food to evaporate the water present than is needed to transport the vapor from the chamber. If the air entering is not dry, or if air leaving the dehydration chamber is not saturated with moisture vapor, the volume of air required is altered. As a rule, 5 to 7 times as much air is required to heat food as is needed to carry the moisture vapor from the food. The moisture capacity of air is dependent upon the temperature.
The volume of a gas at standard pressure increases 1 273 in volume for each 1.8°F. rise in temperature. Each 27°F. increase in temperature doubles the moisture holding capacity of air.
Heat Required to Evaporate One Pound of Water from Food-As a working figure, 1,100 B.t.u. are required to change one pound of water to vapor at common dehydration temperatures. The heat of vaporization is actually temperature dependent.
Volume of Air to Supply Heat to Evaporate One Pound of Water from Food-The volume of air required to evaporate one pound of water is dependent on -the temperature. One cu. ft. of air at 60°F. required 0.018 B.t.u. to increase 1°F. in temperature; one cu. ft. of air releases 0.018 B.t.u. in cooling 1°F.
The heat necessary to evaporate one pound of water vapor at 60°F* and raise its temperature to 160°F. is the sum of the heat of vaporization (1,100 B.t.u.) plus the sensible heat requirement to raise the temperature of one pound from 6Q to 160°F. (100 B.t.u.), or approximately 1,200 B.t.u.
Then the volume of air required to evaporate one pound of water under this condition is equal to the B.t.u. requirement divided by the B.t.u. capacity of air or 1,200/0.018 = 66.700 cu. ft. of air dropping 1°F., or 1,100 cu. ft. of air dropping 60°F.
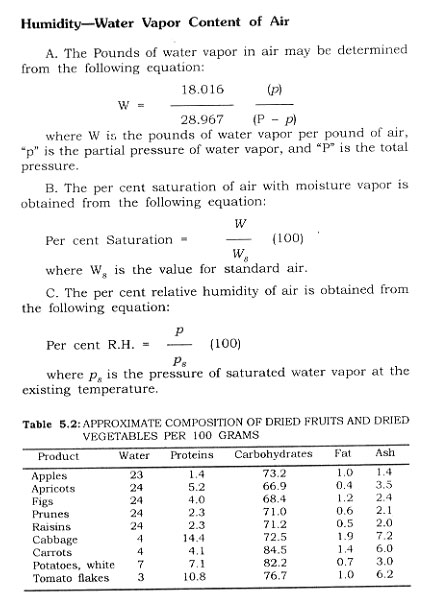
Air Velocity-The amount of heat carried by air in a dehydration chamber can be calculated. At standard pressure, the specific heat of dry air is 0.24 and that of water vapor is 0.47. The volume of air required in cu. ft. per minute to yield a specific number of B.t.u. is as follows:
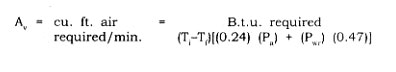
where T. is the initial air temperature, Tf is the exit air temperature, Pa is the pounds of dry air per cubic food, and Pw is the pounds of water vapor per cubic foot.
A minimum of 250 cu. ft. of air per min. per sq. ft. of drying surface is necessary to yield good results in tunnel type dehydration units. An velocities ranging from 300 to 1,000 linear ft. per minute are commonly employed.
Rate of evaporation from Free Surfaces-The greater the surface area the more porous the surface, the higher will be the drying rate of food. The drying rate increases as the velocity of air flowing over food increases. The higher the temperature of air, and the greater the temperature drop, the faster the rate of drying will be, providing case hardening does not develop. Almost as much time may be consumed in reducing the final six per cent moisture as is required to bring the moisture content from 80 down to 6 per cent. The drying time increases rapidly as the final moisture content approaches its equilibrium value.
The rate of evaporation from free surfaces may be estimated from the following equation:
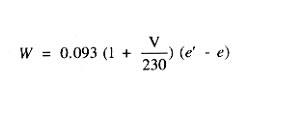
where W is the pounds of water evaporated from a surface per sq. ft. per hour, V is the lineal velocity of air over the surface in ft. per minute, e' is the vapor pressure of water at the temperature being investigated, and e is the vapor pressure of water in the atmosphere.
At 230 ft. per min., drying times are twice as rapid as in still air. At 460 ft. per min. drying occurs three times more rapidly than in still air.
Case Hardening-If the temperature of the air is high and the relative humidity of the air is low, there is danger that moisture will be removed from the surface of foods being dried more rapidly than water can diffuse from moist interior of the food particle, and a hardening or casing will be formed. This impervious layer or boundary will retard the free diffusion of moisture. This condition is referred to as "case hardening." It is prevented by controlling the relative humidity of the circulating air, and the temperature of the air.
Types of Driers-There are many types of driers used in the dehydration of foods, the particular type chosen being governed by the nature of the commodity to be dried, the desired form of the finished product, economics, and operating conditions.
The type of driers and the products upon which they are used are generally as follows:
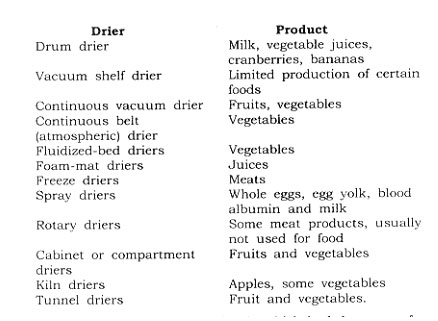
Dehydration is an operation in which both heat transfer and mass transfer take place. Heat is transferred to the water in the product and the water is vaporized. Then the water vapor is removed. Driers can be divided into two classes:
Adiabatic driers in which the heat is carried into the drier by a hot gas. The gas gives up heat to the water in the food and carries out the water vapor produced. The hot gas may be the product of combustion or heated air.
Heat transfer through a solid surface where the heat is transferred to the product through a metal plate which also carries the product. The product is usually held under a vacuum and the water vapor is removed by a vacuum pump. In some cases the product is exposed to the air and the vapor is removed by circulating the air.
It is possible to supply the heat by infra-red, dielectric, and microwave heating methods.
Adiabatic Driers
Cabinet Driers-The drier consists of a chamber in which trays of product can be placed. In large driers, the trays are placed on a truck for ease of handling; in small units, trays may be placed on permanent supports in the drier. Air is blown by a fan past a heater (usually finned steam coils) and then across the trays of material being dried.
The cabinet drier is usually the least expensive drier to build, is easy to maintain, and is quite flexible. It is commonly used for laboratory studies in the dehydration of vegetables and fruits, and in small scale and seasonal commercial operations.
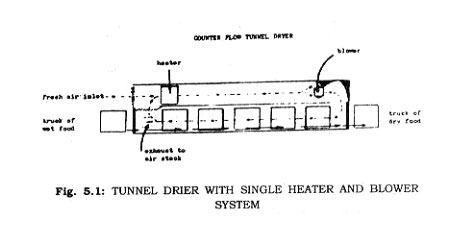
Tunnel Driers-These driers are the most common in use for dehydrating fruits and vegetables. They consist of tunnels 35 to 50 feet long into which trucks containing the trays of food are placed. Hot air is blown across the trays. Production is scheduled so that when a truck of finished product is removed from one end of the tunnel, a truck of fresh produce is put in the other end.
Air movement may be in the same direction as the movement of the product (parallel flow). This has the advantage that the hottest air contacts the wettest product, therefore hotter air can be used. On the other hand, the air at the outlet and becomes cool and moisture laden and the final product may not be sufficient dry.
The air movement may be in the opposite direction of the material flow. In this case, the hot dry air contacts the driest product first so that a very dry product can be obtained. Care must be taken not to overload the drier as the moist charge may stand in the warm, moist air too long without being dried to any extent. This would allow time for product spoilage. On the other hand, the dry product should not be left in the drier too long since it is in contact with the hottest air and could become overheated. In general, the counter flow tunnel uses less heat and produces a drier product than a parallel flow tunnel.
In some cases the two types of tunnels are combined into one unit. The product is first placed in an parallel tunnel to take advantage of the high initial rate of drying in this type of tunnel. It can then be placed in a counter current tunnel to get a very dry end product.
In operation of these tunnels, the drying condifidns are* not constant. When a fresh tray of material is put into the tunnel, the air which reaches the air-exit-end of the tunnel may be cooler and wetter at the beginning of the cycle than at the end of the cycle. There will be a rise in the air temperature and a drop in the moisture content as the product at the air-inlet-end is dried.
In some tunnels a moving conveyor is used instead of trucks and trays. This has the advantage of reducing labor cost and of having more uniform drying conditions. However, a larger installation and investment are required.
Kiln Driers-These are commonly two-story buildings. The floor of the upper story is composed to narrow slats, on which the food product is spread. Hot gas is produced by a furnace or stove on the first floor and passes through the product by natural convection or with the aid of a fan. The material is turned and stirred frequently and a relatively long time is required for drying. Kiln driers are used for drying such products as apple slices, hops, and occasionally for potatoes.
The above driers are generally used to dehydrate relatively large pieces of material. The rate of drying is affected by the properties of the drying air and the properties of the solid. The important properties of the air are temperature, humidity, and velocity. The properties of the solid to consider are the type and variety of vegetable or fruit, the free moisture content, the method of preparation prior to drying, the shape and size of the piece.
It has been found that the drying process can be divided into two parts: the constant drying rate period and the falling rate period. During the former, the rate of drying is governed by how rapidly the air can supply heat to the water in the food particle and remove the water vapor produced. During this period, the water is diffusing to the surface of the particle as fast as it can be evaporated. The temperature of the particle is generally the wet bulb temperature of the air in contact with the piece. However, a point is reached where the water can no longer diffuse to the surface as rapidly as it is evaporated. Then the rate of drying is controlled by the rate of diffusion. As the moisture content decreases, the rate of diffusion drops and the rate of drying slows. The solid material in the particle begins to absorb heat from the air and the temperature of the piece begins to approach the dry bulb temperature of the air.
The rate of drying during the constant rate period is primarily governed by the properties of the drying air. As the water in the solid absorbs heat from the air, the air is cooled. Since the water in the solid is at the wet bulb temperature, the heat available is determined by the difference between the wet bulb and dry bulb temperature of the air rather than the absolute temperature of the air. The vapor pressure of the water in the solid is that for water at the wet bulb temperature of the air, while the vapor pressure of the water in the air is lower. The difference in the two vapor pressure determines the rate at which water vapor can be absorbed by the air. Therefore the air cannot be cooled to the point that it cannot absorb the water vapor produced. The air velocity is important because the more air available per unit of time, the more heat there is available, and the more water that can be carried away in a given time. Also, the heat and mass transfer coefficients are a function of air velocity.
The difference between the wet bulb and dry bulb temperatures (wet bulb depression) governs that rate of drying for a given air velocity. The greater the wet bulb depression, the greater is the rate of drying. For a given wet bulb depression, the greater the velocity, the more rapid is the rate of drying. The rate of drying is also influenced by the method of loading the tray, since this affects the contact between the air and solid food particle. The shape of the solid has some effect since it determines the ratio of surface area to weight of the solid.
During the falling moisture loss rate period, the rate of drying is determined by the rate at which the water at the center of the food particle diffuse to the surface. The nature of the solid and the thickness of the food product are important. It is assumed that the surface of the product is at a moisture content which is in equilibrium with the drying air. This equilibrium moisture is called the critical moisture. The center of the piece has a moisture content higher than the critical moisture. The difference between these moisture levels is the driving force for diffusion. As this difference decreases, 'the"* rate of diffusion drops and therefore the rate of drying falls.
As water leaves the solid, it leaves voids in the solid which shrinks. At low drying temperature, the outer surfaces of a particle shrink inward, producing a wrinkled appearance. This reduces the surface area. At higher temperatures, outer surfaces dry fast enough to form a tough outer shell which resists the forces pulling inwardly. In this case, the relatively soft inner portions of the particle are drawn to the outer surface, leaving a hollow center.
When the outer portion of the particle becomes drier than the center, the soluble sugars are in a more concentrated solution than the sugars in the wet center. This may establish a concentration gradient which causes the sugars to diffuse to the center of the piece. This may lead to darkening, because of browning of the sugars.
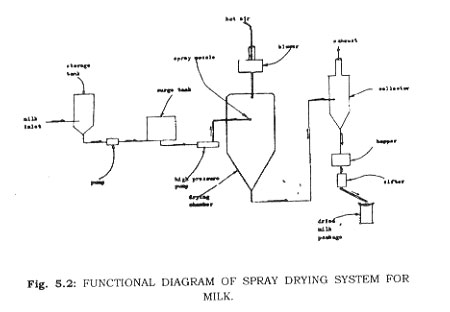
Spray Driers-These are adiabatic driers and many of the considerations for adiabatic drying of solids can be applied to spray driers. They differ in that they used to dry solutions, pastes, or slurries. The food product is not carried on a tray or support, but is dispersed as small droplets which are suspended in the drying air. These have the advantage of very short drying times, and if properly operated, a large portion of the flavor, colour and nutritive value of the food is retained (Fig. 16).
Horizontal Con-Current-The drier consists of a long chamber. The product and the drying air are injected at one and of the chamber. The dried powder settles to the floor, where it is removed by a conveyor. The type is easy to operate, and uses relatively low air velocities. The drying time is limited by the trajectory of the injected particles. The size of the finished particles is therefore limited.
Vertical Down/low Con-Current-In this drier the hot gas and food product are introduced at the top of a tower and travel downwards. The powder collects at the bottom of the tower. This drier is very flexible but usually is a large installation.
Vertical Up/low Con-Current-The hot gas and food product can be introduced into the bottom of a chamber and travel upward. The dry product falls back to the bottom of the drier; the wet gas goes out the top. This type of drier is used where fine, quick drying materials are being handled. The cost is low and unit is small.
Vertical Upflow Counter-Current-The hot gas is introduced at the bottom of the drier and the product at the top. This type of drier is not commonly used for food because the hottest air hits the driest product, which becomes overheated.
Mixed Flow-The food product is introduced at the top of the drier. Hot gas is introduced at the top of the drier such that it follows a spiral path to the bottom of the drier. This allows a longer path for the particles to travel for a given tower height. A drier can be designed to have the gas spiral back up the drier and eject the powder from the top of the drier.
The hot gas used for drying can be the direct product of combustion or heated air. The food products may be sprayed in with nozzles or atomizers. One-fluid nozzles are the most commonly used, being cheap and highly flexible. Centrifugal atomizers are used for pastes and materials which plug nozzles. The dried product collects on the floor of the drier, or may be collected outside the drier in bag filters or cyclones.
Air Lift Driers-Special driers have been used to producing such foods as potato granules. The fresh, moist, granular potato particles are mixed with hot air and carried up a narrow column. The air velocity is such that the granules are suspended in the air stream as they dry. At the top of the drying column, the product and air enter a section where the air velocity drops enough to permit the dried particles to settle into a collector. The wet air goes out the top of the drier.
Foam-mat Driers-These are used primarily for liquids which are pre-foamed by whipping, adding a low level of an edible whipping agent to liquids that do not whip readily. Foaming a liquid exposes enormous surface areas for quick moisture removal which also permits use of lower drying temperatures. Foam is deposited in a uniform layer on a perforated tray or belt through which hot air is blown. Foam layers of many foods can be dried to about 2-3% moisture in approximately 12 min.
Heat Transfer Through a Solid Surface
Drum Driers-Steam heated rotating drums 2 to 6 feet in diameter are used for dehydration of fluid products. The slurry is deposited on the drum in a thin film. Heat is transferred through the drum wall to the product film. The drum may be exposed to the atmosphere or it may be held under a vacuum. The dried product is removed from the drum by a scraper blade. The dried film then may be ground to a fine powder (Fig. 5.3).
Vacuum Shelf Driers-These consist of a cabinet with hollow shelves. The product is placed in pans on the shelves, or if solid, it can be laid directly on the shelves. The unit is closed and a vacuum drawn. Steam, hot water, hot oil, Downtherm or some other suitable heating medium is circulated through the hollow shelves heating the product. These units are expensive and have been used mainly on such product as "puff-dried" citrus powders, tomato powder, and other food products.
PRINCIPLES OF FOOD PRESERVATION BY CANNING
The Art of "Appertizing"
Man is unable to solve problems he does not know exist. Inventions are combination products of observations and items of memory. An invention is usually composed of several parts which are known but unorganized, plus one or more parts, which, when added to previously known information, create a concept unrealized prior to the invention. Canning as known today is the product of such a process.
France in the late 1790's was at war and having difficulty feeding its people. Napoleon's fighting forces had a diet of putrid meat and other items of poor quality. The foods available couldn't be stored or transported except in a dry state. Recognizing an important problem, a prize was announced offering 12,000 francs and fame to anyone inventing a useful method of food preservation.
Nicolas Appert, a French confectioner, working in a simple kitchen, observed that food heated in sealed containers was preserved if the container was not reopened or the seal did not leak. He modestly called the process "the art of Appertizing." Appert received the award from Napoleon after spending ten years proving his discovery.
It should be appreciated that the cause of spoilage of food was unknown. The great scientists of the day were summoned to evaluate Appert's process and offer explanations for its apparent success. The conclusion reached was that the process was successful because in some mysterious and magical fashion air combined with food in a sealed container, preventing putrefaction. This was quite incorrect. Nevertheless, the preventing putrefaction. This was quite incorrect. Nevertheless, the canning process was discovered and practiced for the next 50 years with some success, but in the darkness of ignorance.
Canning from 1800-1850. Appert began work on his process in 1795. Peter Durand received patents in England in 1810 for glass and metal containers for packaging foods to be canned. The tin-plated metal containers were called "canisters" from which the term "can" is assumed to be derived. Early metal contains were bulky, crude, and difficult to seal. By 1823 a can with a hole in the top was invented, allowing the food to be heated in boiling water baths with the hole covered with a loose lid. The lid was soldered into place after the heat treatment. Hole-in-top cans are in use presently for canned evaporated milk, although the cans are sealed prior to heating.
By 1824, Appert had developed schedules for processing some 50 different canned foods. Meats and stews processed by Appert were carried by Sir Edward Perry in 1824 in his search for a northwest passage to India. Several cans of food from this voyage were obtained from the National Maritime Museum in London in 1938 and opened. The food was found non-toxic for animals. Interestingly there were isolated from these canned products bacteria which had been dormant for at least 114 years. Given proper environment and substrate, they grew!
In the 1820's canning plants appeared in the United States in Boston and New York. By 1830 sweet corn was being processed in Maine. By 1840 canneries began appearing throughout the United States.
Temperature vs. Pressure of Boiling Water
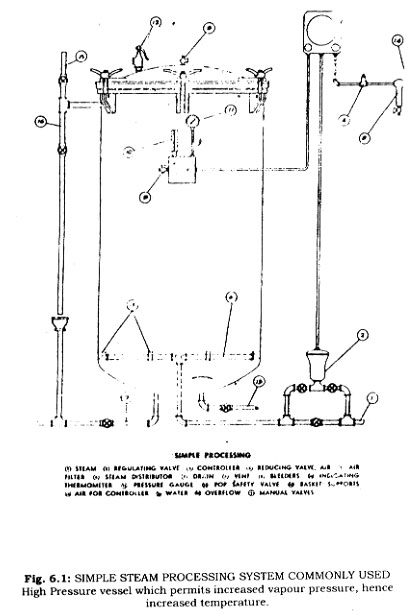
Canning from ,1850-1900. In 1851 Chevalier-Appert invented an autoclave which lessened the danger involved in the operation of steam pressure vessels. It was recognized that some foods could be processed for shorter times if higher temperatures were available. It was learned that the temperature of boiling water could be increased by adding salt. Demands for greater production in factories could be met if the cooking times for foods could be reduced. For instance, the boiling water bath cooking of canned meats could be reduced from six hours to perhaps a half hour by cooking the cans in a water-calcium chloride solution. Production could be increased thereby from some 2,000 cans per day to 20,000 cans per day. Losses are to failure of containers were large. No pressure was applied to the cooking vessels. Commercial cans were unable to withstand the internal pressures developed by heating to 240°F.
The temperature at which water will boil is depending upon the pressure. Using a pressure vessel, it was possible to achieve temperatures in the vicinity of 240"F. However, these retorts were still dangerous to operate.
Spoilage of Food Caused by Micro-organisms
In 1862 President Lincoln signed the Morrill Act, creating the land grant colleges (Purdue, Michigan, Massachusetts. Illinois, etc.). The great scientific debate in universities at that time was "spontaneous generation" of life. At this time, too, Louis Pasteur, son of a well-decorated officer in Napoleon's ' army, became interested in the problems of the great wine and beer industries of France which were threatened with ruin; their products were diseased and souring from "spontaneous generation" of life in bottles and kegs.
To the Academy of Sciences in France in 1864, Pasteur reported that he had found the cause of the disease of wine and beer to be a microscopic vegetation. When given favorable conditions, this vegetation grew and spoiled the products. However, boiled wine sealed from contamination in jars with even cotton plugs would not sour. In fact, it was possible to isolate this microscopic vegetation from the cotton plugs! It was this microscopic growth which spoiled foods, and it was necessary for such organisms to gain entrance to heated foods if they were to spoil! Here was an explanation for the success of Appert more than half a century before. The concept of heat treating foods to inactivate pathogenic organisms is termed appropriately "pasteurization" today.
It is interesting to note that magnifying lenses were used by Bacon in the late 1200's, but had never been focused on a drop of water until the 1600's by Leenwenhoek. He had noted microscopic growth which he named "animalcules," but they were only a curiosity in water to him. Two more centuries elapsed before this information was organized and synthesized into an explanation for "spontaneous generation" of life.
Appert had established that containers of food must be carefully sealed and heated. Cleanliness was important to his process, although he did not know that micro-organisms were the agents of spoilage. Pasteur established several important principles. Most changes in wine depended on the development in it of micro-organisms which were themselves the spirits of disease. Germs were brought by air, ingredients, machinery, and even by people. Whenever wine contained no living organisms, the material remained unchanged. Some of Pasteur's flasks remain, and are presumably still sterile today.
From Appert's work the term "hermetic came into use, meaning a seal such that it sealed out spirits and ferments. Foods treated with heat in hermetically sealed containers are called canned foods. The seal is important not only to prevent the reinfection of food but also to prevent the transfer of gases.
By 1874 Shriver invented a pressure steam retort which had control features. It became adopted quickly by the canning industry. A simple steam pressure retort such as is commonly employed.
To recall the food carried by Perry in 1824 for a moment, the cans opened in 1938 contained viable bacteria. In the 1800's many foods were spoiling after being canned, just as we find many occasional instances of spoilage today. Prior to the turn of the 20th century, it remained to be established that foods heated to nearly 240°F could be spoiled from under heating. Certain organisms are very heat resistant and capable of spoiling canned foods even after being heated to such a degree. In addition there are heat resistant bacteria which can grow at very high temperatures (170°F.). If cans are not cooled promptly, these heat resistant thermophilic organisms may survive the heating process, then grow and spoil the canned food. Foods to be stored in warm or hot areas require special attention.
Canning from 1900-1950. In 1906 the Food and Drug Act was signed. This was a milestone of progress in social legislation and has been considered by many to b^e a landmark of accomplishment in food technology. Together these have given the United States the best food supply in the world. However, the task is by no means completed; new developments create new problems.
With the 20th century came the common "sanitary" tin can of today. It should be recognized that the term "tin" can is a misnomer. Actually the container is a tin coated steel can, having from 0.25 to 2.0 per cent tin. By the time of World War I the sanitary can was in general use, with high speed can making and sealing machines. In 1921 commercial production of cans with enamelled inner surfaces was underway.
The 1920's saw a host of researches actively evaluating the canning process. Information was accumulated on the heat resistance of bacterial spores, on heat penetration through the contents of cans, and even a mathematical solution to the problem of time-temperature processing schedules for canned foods was evolved by C. Olin Ball. Process times and temperatures were put on a sound basis, replacing the trial and error methods of the past.
In the 1930's much research was devoted to the study of nutrients important to man. Vitamins were better understood and new ones were being discovered. These and other information about food and human nutrition were applied in the food preservation industries, with resulting improvements in the nutritional value of processed foods.
From the nutrient supply standpoint, in World War II the troops of the United States were the best fed in any war. No one would argue that the ultimate had been reached. Cold meat and vegetable stew were nutritious, but left something to be desired when eaten cold from the can. Napoleon said that an army travels on its stomach. It is still true.
During World War II it was possible to evaluate some of the changes in male population characteristics since World War I. The sons were taller than their fathers had, been., %nd generally evidenced some of the promises of the goal of the science of human nutrition. Canned foods no doubt contributed to this improvement, providing a more varied and balanced diet throughout the year, within the reach of most of the population.
Canning from 1950. The canning industry in recent years has made steady progress in the area of the mechanical efficiency of processing plants. More production is obtained with fewer people. Some factories produce a million or more cans of food a day. Yet the food industry is not apt to be compared with chemical and petroleum industries in plant efficiency and control.
Recent canning developments have been in agitation retorting; contents of cans can be heated at increased rates. In aseptic canning the food and container are sterilized separately, then meet at sterile filling units to be subsequently sealed in sterile chambers. Sterile canning techniques have been found valuable in preserving many heat sensitive foods (banana puree, milks, etc.). There are inherent difficulties maintaining sterility at all points in the system, and holding sterile products during equipment breakdown.
The food preservation practices prior to the discovery of canning were copied from nature. Canning has no counterpart in nature. Canning is a method of controlling natural processes. Canning is a capital invention, which has changed the eating habits of the western world.
The more man is able to understand the world in which he lives, the more uses he finds for his new information for the betterment of mankind. It has been 150 years since the discovery of the "art of Appertizing," 100 years since Pasteur's discoveries, 50 years since thermophilic bacteria were discovered in canned foods. The last word has not been written on the process, and the last discovery has not been made. The mechanism of death to micro-organisms is not understood today. Who amongst us would dare say that opportunities similar to those of Appert are no longer available?
Evolution of Containers for Canning
The container is important to success in the canning preservation of foods. While Appert used glass containers in his canning experiments, the tin coated steel can has been used largely during the past hundred years by the commercial canneries. Each container has certain rather exclusive uses. Glass is the traditional container for jams, jellies, preserves, green olives, and various pickled products. In the household the glass jar is used to the practical exclusion of tin for home preservation of fruit, vegetables, meats, preserves, etc. Glass is commonly used for such items as fresh milk, catsup, carbonated beverages, juices, and beer. At present one container is invading the other's field; thus much beer is now packed in cans, and glass containers that will withstand agitation sterilizers are used. Either container is adequate. There is promise for the future of tinless tin cans, and the glass industry has experimented with tin coated glass jars. Tin has protective qualities to food in containers, although it will bleach fruit colors. Glass is an inert container, although damage to food may result from the action of light-instigated reactions. The selection of one container over the other is usually decided on the basis of process and product.
Glass Containers. Glass is defined as a mutual solution of suitable silicates formed by heat and fusion, with cooling to prevent crystallization. Glass is an amorphous, transparent or translucent, supercooled liquid; investigations have failed to reveal the existence of crystalline components in glass.
Glass was known early in history, at least in 1600 B.C. The first glass was made by mixing sand with the ash of seaweed, covering with clay, and heating in a hot fire. In the period of the Roman Empire glass was melted and blown into bottles and fixtures. The next change in glass jar making involved blowing molten glass into molds to form the object. First wood and later iron molds were used. In 1880 a glass bottle machine was invented which used compressed air instead of human lung power to blow glass.
Glass usually consists of three types of oxides: 1) The glass-forming oxide of silica (high grade sand). Certain phosphates are also glass forming materials. 2) The fluxing oxides. Sodium, potassium, or lithium oxides are used, the first predominating. Fusion of fluxing oxide with the glass forming oxide yields a product soluble in water. 3) Totlecrease o"-this solubility, a third group of oxides known as stabilizing oxides are used, generally calcia and magnesia. Barium oxide and alumina are used to lesser extents.
Glass food containers consist of the silicates of sodium, calcium, and magnesium. The approximate composition of glass for fruit jars is as follows: SiO2 74 per cent; Na2O 18 per cent, CaO 7 per cent. MgO 1 per cent, and traces of Fe2O1 and MnO2.
In making glass the ingredients are weighed and mixed with cullet (broken glass), in a revolving mixer. The cullet has the same general composition as the glass to be made, and melts at a lower temperature than the other ingredients, assisting in the liquefication of the batch of ingredients. When the cullet and raw materials are thoroughly mixed they are transferred to a melting furnace, where the temperature is maintained near 2600°F. After the fusion of ingredients has occurred and gases are expelled, the batch is allowed to cool until it reaches the desired viscosity. Glass containers are made from the conditioned molten glass. Glass technology is a highly specialized field and outside the scope of this text. Glass jar making equipment is automatic and in the United States either Owens-type or flow-type processes are used. The Owens process has a mold which scoops molten glass from a reservoir by drawing a gob of glass into the blank mold by vacuum. The mold lifts, and a knife edge shears excess glass from the mold. The neck of the bottle is formed by the neck or ring mold. The blank mold opens, a mold containing the desired contour of the bottle rises, closes about the gob of glass, air is applied through a blow head above the neck ring supporting the gob of glass, and it is blown into the shape of the mold. The blow head is removed and the bottle is discharged to a conditioning chamber.
In the flow-type system a gob of molten glass is dropped into a blank mold where the neck is formed by pressure. The mold opens and the partially formed bottle is transferred to the finishing mold which has the desired contour. Air pressure is applied, the gob of glass forms against the mold, the mold opens, and the formed bottle is discharged to the conditioning chamber.
In either system the formed glass container is cooled for an hour or two to control brittleness. Strains in glass are controlled by the temperatures of the conditioning chamber. After this annealing process, the bottles are discharged, inspected and packed.
Ordinary flint glass is decolorized by the addition of small amounts of cobalt or selenium oxides to the batch. Light green glass is prepared by adding small amounts of iron or arsenic oxides. Emerald green bottles are colored by the addition of chromium salts. Amber glass is colored by the addition of carbon or sulfur or a combination of iron and manganese oxides. White or milk glass is made by adding fluorides or alumina.
Glass containers are sealed with caps, made from tinplate or aluminium, and lined with cork or paperboard over which a disc of aluminium, tin, paper, plastic, or resin is placed.
Cork comes from the outer bark of a species of oak tree grown chiefly in Spain, Portugal, Algeria, and California. The bark is removed from the tree, dried, softened in boiling water and steam, flattened, and cleaned of its rough outer surface. At the cap manufacturer's plant, the cork is pulverized, mixed with a binder and formed into rods, or sheets. Rods are sliced into discs. Sheets are laminated and punched. The caps are lined with these discs.
The common crown cap of a bottle of soda is the strongest part of the container. The bottle will break before the cap is blown.
Glass containers are sealed automatically. Vacuum sealing involves passing a filled bottle through a short tunnel which is being swept with steam. This serves to sterilize the top surface of the bottle while at the same time displacing the air in the headspace of the bottle.
The bottle moves through the steam chamber, picks up a cap, which is quickly pressed into place. Cooling causes condensation of the vapor at the top of the bottle, leaving a partial vacuum. By adjusting the flow of steam, a vacuum ranging from 2 to 28 inches of mercury may be attained by this process. Containers may also be vacuum sealed with a mechanically produced vacuum.
Caps for bottles may be placed plain on tne bottle,-the screw being formed by machining the plain cap to the bottle. A rapidly rotating head follows the contour of the glass container, forming the screw design in the cap.
Caps for bottles have a sealing function. Caps enforce a seal either at [he top of the neck, at the side of the neck, or at the shoulder of the neck of the bottle.
Tin Containers. Although tin vessels have been used since ancient times, the process of tin plating was invented in the 1200's and was a closely guarded secret until the 1600's. In 1730 there was commercial tin plating in Great Britain. In 1873 commercial production was underway in the United States.
At first iron bars were hammered into thin sheets by hand. An iron oxide film forms on such sheets which must be removed by scouring and soaking in acid. Originally the acid used was the product of fermentation. The term "pickling" iron plate no doubt derives from this process, which means treating with dilute acid.
The cleaned iron plates then were passed through a bath of molten tin. After being tinned, the plates were cleaned and polished with sawdust and moss. Tinning pots were stratified with oil, to prevent oxidation of the tinned surface.
Hammering of iron bars into plates was replaced by rolling the bars through high pressure mills, which flattened the bars into plates.
In the 1800's sulfuric acid replaced the fermented liquids for pickling black iron plate. Bessemer steel replaced iron. A zinc chloride flux came into use which aided the union of tin to the prepared steel plate surface.
In the present century there have been a number of advances in tin container production. The invention of the sanitary can led to nearly automatic production of cans.
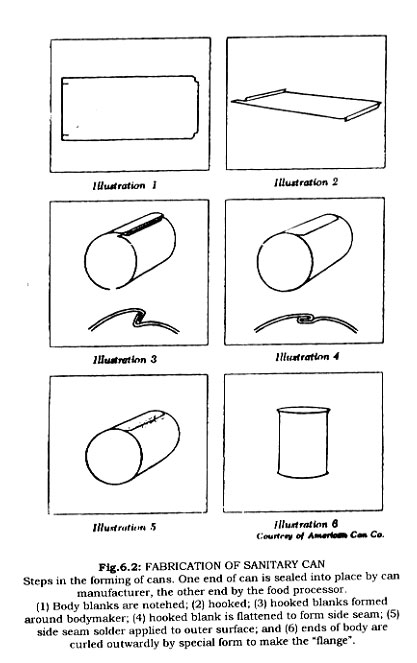
In early can manufacturing, sheets of tinned plate were marked and cut by tinsmiths. The sheets were bent into shape on a roller, the edges of the body blank overlapping, and solder was applied. End discs were cut larger than the body of the can. The edges were turned to form a flange, into which the body blank fit snugly, and was soldered into place. Under favorable conditions it was possible for a tinsmith to make a hundred cans a day. Modern equipment manufactures this number in 20 seconds or less. In present production sheets of tin plate are fed into a machine, which slits and trims the sheets into the lerigth and width of the body. The trimmed sheets are notched at the corners, the edges are hooked, the blank bent into a cylinder, the hooks engaged flattened and the locked seam soldered. The ends of the body are flanged to receive the top and bottom of the can. Can ends are punched from sheets. The edges of the lids are curled, then a rubber-like sealing compound is flowed into the curl, automatically. The can bottom is then attached. After pressure testing to insure absence of leaking, the cans are shipped to canneries where food is packed into the cans and the top is sealed into position. Cans are expected to have less than one defective per ten thousand.
Highly colored foods bleach in tin containers, but retain their color in lacquered cans. The lacquer is applied and baked to one side of tin plate, then the plate is formed into containers.
Tin cans are made in a great variety of sizes and shapes, developed by trade customs rather than by the needs of consumers. The canner refers to the size of a can by symbols, i.e., 211 by 400. This means that the can is 2-11/16 inches in diameter and four and no 16th inches high. The first number denotes the diameter and the latter the height of the can. the first digit is in inches, the last two digits denote the number of 16th. A 307 by 409 can is 3-7/16 inches in diameter and 4-9/16 inches high. The following are a few examples of common standard cans:
| Name | Cans | Capacity in Ounces of at 68°F |
| No.1 |
211 X 400 |
10.94 |
| No.2 |
307 X 409 |
20.55 |
| No.2*1/2 |
401 X 411 |
29.79 |
| No.3 |
404 X 414 |
35.08 |
| No.10 |
603 X 700 |
109.43 |
The trend is towards smaller container size whereas the No. 2 can was used for many consumer procjucts for generations, it is being displaced by the 303 can which is smaller. No doubt rising prices and costs have piayed a role in this shift. Can sizes of smaller dimensions have become increasingly popular, too, for small families and apartment house dwellers of the country. The No. 1Q can is we]j established in hotel, restaurant and institution tracjes
Aluminium containers have recently becorne avaiiable in the United States. Flexible containers are now being tested.
The unit operations in canning are show) generaiiy.
Important Food Groups
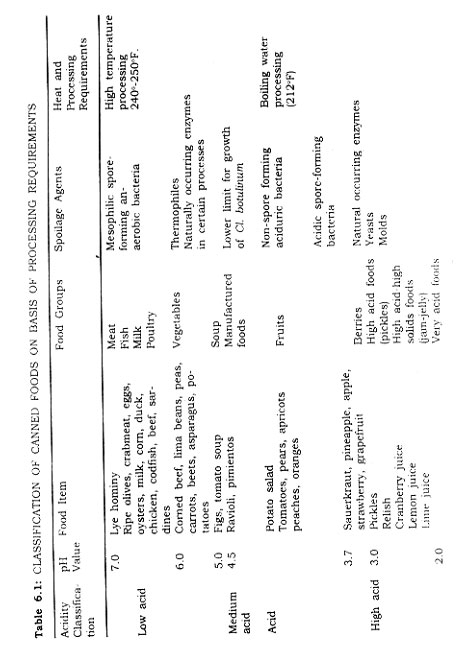
Alkaline Foods. Foods with pH values in the basic range are few, Old eggs, aged seafood, soda crackers, as lye hominy may have pH values higher than 7.0. Lye homily is the only food item canned which is normally over 7.0. Lye degree of alkalinity is dependent upon manufacturing procedures If all the lye is washed from the treated kernels of corn it would be expected that its pH would be slightly less than 7 0
Low Acid Foods. Meat, fish, poultry, dairy products, and vegetable foods of man generally fall into a pH range of 5 0 to 6.8. This large group is commonly referred to as the low acid group, and in some cases these, foods are even referred to as non-acid foods. While they are relatively i)On-acid they do fall in the acid range of pH values.
Manufactured food items such as soups and spaghetti products, as well as figs and pimientos fall into what is called the medium acid food group. These foods.,have pji,values between 4.5 and 5.0.
Foods with pH values greater than 4.5 require relatively severe heat treatments. The lower limit of growth of an important food poisoning organism, Clostridium botulinum, is at a pH value of 4.5 Inasmuch as a millionth of a gram of the toxin produced by this organism will kill man, certain precautions are indicated. All foods capable of sustaining the growth of this organism are processed on the assumption that the organism is present and must be destroyed. Foods could be classed into two groups, depending on whether this organism can grow or not.
Acid Foods. Foods with pH values between 4.5 and 3.7 are called acid foods. Fruits such as peaches, pears oranges, apricots, and tomatoes fall into this class. Potato salad made with vinegar is also in this group.
High Acid Foods. Next in order of increasing acidity are the berries, pickle products, and fermented foods. The pH values range from 3.7 down to 2.3. An example of this high acid group is cranberry juice.
Another important group of foods is one termed high acid-high solids. Jams and jellies are in this classification.
It is possible to classify the foods of man then on the basis of acidity and pH value. Plant tissue (except fruits and berries) and animal tissue (including meat, fish, and dairy products) are classed as low acid foods. Manufactured items with several ingredients may fall into the medium acid group. Fruits are in the acid group. Berries, fermented products, and certain citrus products fall into the high acid group as do jams and jellies. Few foods are basic in reaction if considered in their best quality.
A summary of the acidity classification of foods is presented in Table 6.1.
Micro-organisms Associated with the Food Groups
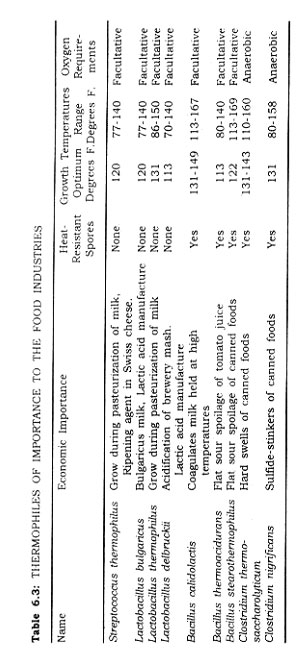
Most micro-organisms if actively growing (vegetative state) are readily killed by exposure to temperatures near the boiling point of water. Bacterial spores are more heat resistant than vegetative cells. As mentioned previously, bacteria can be classified according to their temperature requirements for growth. Bacteria of the soil, water, air, and body growing at room temperature or slightly higher are called mesophiles; their range is between 70°F. and 110°F. Some water and soil type bacteria grow best at temperatures ranging from 35°F. to 50°F., and are called psychrophiles. There are bacteria of soil, water, and air which grow best at temperatures from 120°F. to 170°F., and the called thermophiles.
It is important to distinguish between organisms capable of growing at moderately high temperatures (150"F.), the thermophilic group, and those capable of resisting the effect of high temperatures, the thermoduric group. Mesophilic organisms can be thermoduric due to their spores, as can the spores of thermophilic bacteria.
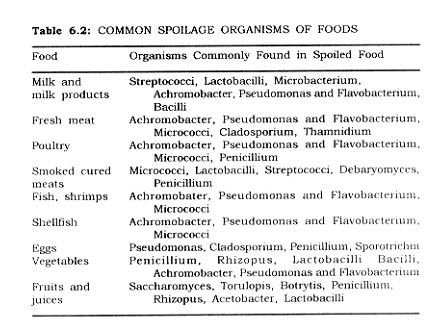
Typical genera of micro-organisms commonly associated with the spoilage of important foods are given in Table 6.2. The thermophiles of importance to the food industries are presented in Table 6.3, along with other information pertinent to the canning process.
Foods have associated microfloras; certain organisms are associated with particular food groups. These organisms gain entrance to the food during the canning operation either from the soil, from ingredient, or from equipment. On the basis of the acidity classification of foods, it is possible to make general statements relative to the spoilage organisms encountered of importance to the success of the canning process (Table 6.1).
Micro-organisms of Low Acid Foods. In foods with a pH value greater than 4.5, mesophilic spore-forming anaerobic bacteria are important, see Table 6.1. Clostridium botulinum is a soil borne mesophilic spore-forming, anaerobic bacterium. Another is one known as Putrefactive Anaerobe (P.A.) No. 3679, a Clostridium sporogenes type, common in soil. The latter is more heat resistant than the former, and is used to evaluate many heat processing schedules. If heating is adequate to kill the spores of P.A. No. 3679, the process insures the destruction also of Clostridium botulinum.
In addition to the mesophilic spore-forming bacteria there are also thermophilic spore-forming organisms, which are very heat resistant. In fact, they may be more heat resistant than the mesophiles. Processes designed to kill all thermophilic spore-forming bacteria may also result in canned foods far overcooked and degraded in nutritional value. These organisms therefore are controlled through sanitation and by strict control of ingredients, which may be highly contaminated. For example, it is nearly impossible to sterilize chocolate with moist heat, due to the high fat content of the chocolate which apparently entraps (thermophilic) organisms, causing death by dry heat conditions rather than by moist heat, Canned products (i.e., chocolate milk) containing such ingredients may be especially difficult to sterilize.
Micro-organisms of Acid Foods. In the acid food grouping, the troublesome organisms are aciduric bacteria of no special heat resistant qualities. Bacteria, yeasts, and molds are capable of spoiling these foods. The lack of growth of Cl. botulinum in acid foods is reflected in their low heat processing requirements. A few mesophilic anaerobic spore-forming organisms (i.e., Cl. pasteurianum) may cause spoilage, but in acid foods the organism has relatively low heat resistance. Bacillus thermo-acidurans is an exception worth noting. It is the flat sour spoilage organism of tomato juice, Canned tomatoes generally are not found spoiled by this organism, although it could spoil this product. Thermophilic flat sour spoilage is due to an inoculation of food by equipment or ingredients and is related to the sanitary condition of a plant. Flat sour spoilage of home canned tomato juice is not common because equipment in homes is easily cleaned. In flat sour spoilage, as the name implies, acid is produced without gas. One difficulty then is that containers do not appear to be spoiled until open, either by the canner, buyer, or worse, by a consumer.
It is a general axiom of the canning industry never to taste spoiled low or medium acid foods, due to the threat of spoilage by Cl. botulinum. With acid and high acid foods, the spoiled products may be distasteful, but there is reasonably little cause for alarm in their being tasted. It is good practice to give a can of spoiled food due respect, in any case. Fortunately most canned food spoilage is associated with the production of gas, bulging the container. There may be visible signs of decomposition in the canned food itself.
Micro-organisms of High-Acid Foods. Aciduric bacteria, yeasts, and molds are the troublesome organisms in this group. Their heat resistance is generally low. In this group too, the natural enzymes present in food may be as heat resistant as micro-organisms. Heat processes for pickles must give due consideration to the destruction of the natural enzymes of cucumbers. It is no less difficult to destroy enzymes than bacteria, yeast, or molds in pickles.
Sources of Spoilage Organisms
Spoilage micro-organisms troublesome in. canning, are organisms of soil, water, air, and animals, fable 55 shows the common groups associated with various foods.
Organisms of Soil. While all the spoilage types are to be found in the soil, unless there is a transfer of soil (or dust) to the container of food, soil is not an important source of spoilage organisms. There is of course the contamination of raw products taken from the soil, but ordinary processing in a cannery should find soil washed adequately from food. There is no excuse for food containing soil being canned. The surface of certain foods not peeled or prepared for canning will contain varying numbers of soil borne micro-organisms. Asparagus and green beans could be contaminated with soil unless the washing process is adequate. Carrots, beets, and tomatoes, for example, are peeled. The surface contamination of these products would not directly come from soil. Meats are prepared from butchered animals, and soil contamination would be unlikely. Certain animal products such as pig's feet would be an exception, not different from asparagus relative to soil inoculation. Dust carried through air to cans and products may be a source of contamination.
Equipment. Spoilage micro-organisms can normally be expected to be present on equipment, particularly that having a steam connection. When undue spoilage occurs from understerilization, attention should be directed to accumulation of spoilage organisms on equipment. Only micro-biologically, physically, and chemically clean equipment can give desired results. Contamination of foods by equipment means either poor sanitary practices, poor equipment design, poorly utilized equipment, or a combination of these factors. It is not possible to clean constantly equipment during operating periods, and in spite of a sanitation program, it is possible to have clean equipment contaminated by the flow of raw food products. For example, if tomatoes containing rot are not sorted from processing lines outside the factory, these decomposed tomatoes will deposit mold on equipment and mold could be transferred to sound products. In the case of tomato products, microscopic examination of the finished commodity for mold focused as an index of quality. Mold in tomato products is undesirable. According to our laws one decomposed tomato in a hundred sound fruit is adequate to cause the entire lot to be declared unfit for human consumption.
Equipment is a constant source of contamination of food products in canneries. Careful attention to sanitation practices and the condition of raw products flowing through a plant are essential to successful canning preservation of foods.
Ingredients. Sugar and starch are common ingredients in foods and have been found to be sources of mesophilic and thermophilic spore-forming bacteria as well as other microÂorganisms. Standards have been established for these ingredients by the canning industry. Other ingredients including dry milk solids, syrups, flours, and spices are generally contaminated with micro-organisms. Packing brine for canned sweet corn or peas prepared from sugar having 40 flat sour spores per gram when made into the packing solution could yield 500 or more spores per can! Hence careful attention is indicated to ingredients which are added to foods during preparation and canning preservation.
Botulism. Due to the public health significance of Clostridium botulinum, it is worthwhile to discuss this organism more thoroughly.
It is possible that at one time or other many people have eaten this bacterium, as it is a common soil organism. There are at least four types, two of which (A and B) are noteworthy here. Type A occurs generally only in virgin soil or newly reclaimed forest areas. Type B is found in cultivated soils.
This organism is a gram positive, anaerobic, spore-forming bacterium. It develops only in the absence of atmospheric oxygen, or in low oxygen tension environments.
The organism exists in both a vegetative form and in the form of a heat-resistant spore. The vegetative form is easily killed by moist heat at temperatures below 212°F. The spores on the other hand may survive 300 minutes of boiling at 212"F. The spores are the important form of the organism from the canner's or food processing viewpoint. Spores are found in dust-and soil. The main contamination is from the soil in which food is grown. Most animals become cotaminated, too. It is a difficult organism to work with because it loses its toxin producing capacity when manipulated in laboratory media. Spores vary in heat resistance, and if is not srfriple to obtain a spore suspension of uniform heat resistance to study.
The organism has both proteolytic and saccharolytic powers. The media in which a culture actively is growing has an odor much like decomposed meat mixed with butyric acid. However, it is not unlikely to find the organism present, with its toxin, and still have the material reported as not objectionable to taste or smell. Hence, persons ingesting such food who are not killed may not suspect the food from which they became poisoned. Cl. botulinum has a heat resistance at 250°F. of 2.8 minutes for 10,000 spores per ml. in neutral phosphate buffer solution.
From a public health standpoint, foods with pH values greater than 4.5 must be processed such that the sterilization value is equal to that of 2.8 minutes at 250"F. As noted previously, there are other organisms with greater heat resistance which must be destroyed if the food is to keep at ordinary storage temperatures.
The botulina toxin produced is water soluble, and extremely lethal to man. It has been characterized chemically recently by researchers in the United States.
The spores must germinate in order to produce a vegetative cell to produce the toxin. The toxin is destroyed by a ten-minute exposure to moist heat at 212°F.
Type A and type B toxins are important to man, and it is possible to determine which type toxin is present by antigenic reactions.
A food suspected to contain Cl. botulinum spores may be exposed as follows: The sample is inoculated into suitable sterile media (liver broth), given anaerobic conditions (mineral oil layer, agar layer) and incubated at 97°F. At the end of 72 hours, the medium is filtered and one-half ml. units of this filtrate are injected into mice. Antitoxin A or B is administered to a portion of the mice interperitionially. If the toxin is present, some mice will die. If control mice die, those receiving anti-toxin A live, and antitoxin B die, then Type A toxin was present. The causative organism was Clostridiwn botulinwn Type A.
A suitable anaerobic condition for this organism to grow would be a sealed can with a vacuum or deep in a liquid or in the center of inoculated meat products, or in oil packed fish etc. However, it has been reported that aerobic organisms may grow and use up oxygen in a container, creating anaerobic conditions adequate for growth of Cl. botulinum. In an acid product when the acid has been utilized by other (mold) organisms, raising the pH, Cl. botulinum may grow if present.
The boiling of home canned products for 10-15 minutes prior treating is a safety practice which should be carefully adhered to. At the present time some ten people are killed annually by botulina toxin poisoning in the United States.
Accurate time-temperature records must be kept by processors for just this reason. Any serious outbreak of botulism would certainly be detrimental to the food canning industry. Until recently hermetically sealed cans have not been used to package low acid foods to be frozen. Research has indicated that there is no more danger with sealed cans than with any other sealed container. Improperly handled frozen foods present a problem of equal significance.
One important rule should be remembered: never taste low acid canned or frozen foods suspected of being spoiled.
PRINCIPLES OF FOOD PRESERVATION BY FERMENTATION AND PICKLING
LIFE WITH MICRO-ORGANISMS
Micro-organisms no doubt outnumber other living entities on this planet and can be found existing actively or passively wherever living organisms occur. While the energy for life on this planet is captured by green plants in the photosynthetic process, micro-organisms are generally responsible for the final decomposition of the photosynthetic products. Animals play a minor role in the cycle.
In as much as bacteria, yeasts, and molds are to be found throughout the environment of man, it is to be anticipated that these micro-organisms are in direct competition with other living entities for the energy for life. Whenever the conditions of nutrients and environment are favourable for microbial activity, it will be found (Table 7.1).
Man must compete with all other living entities on earth. In order to retain food supplies for himself, he must interfere with natural processes. Through his study, and as a fruit of his curiosity, man has evolved a number of control systems. One is the preservation of food by controlling, yet encouraging, the growth of micro-organisms. Under such a condition, man may employ micro-organisms to create unfavourable conditions for other microbes, yet retain in the foodstuffs the nutrients desired.
While micro-organisms were not identified as the important agents in food spoilage until a century ago, wine making, bread baking, cheese making, and salting of foods have been practiced for more than four thousand years.
For all those years mankind practiced food preservation using unknown, invisible, active, living organisms.
While food preservation systems in general inhibit the growth of micro-organisms, all such organisms are not detrimental, in fact, some are commonly utilized in food preservation. The production of substantial amounts of acid by certain organisms creates unfavourable conditions for others.
To review terms for a moment, respiration is that process whereby carbohydrates are converted aerobically into carbon dioxide and water with the release of large amounts of energy. Fermentation is a process of anaerobic, or partially anaerobic, oxidation of carbohydrates. Putrefaction is the anaerobic degradation of proteinaceous materials.
Sodium chloride is useful in a fermentation process of foods by limiting the growth of putrefactive organisms, and inhibiting the growth of large numbers of other organisms. Certain bacteria tolerate and grow in substantial amounts of salt in solution.
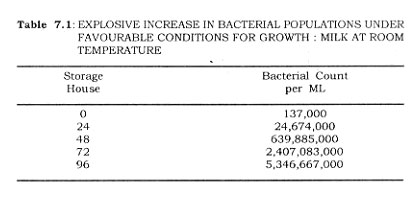
FERMENTATION OF CARBOHYDRATES
The word fermentation has undergone evolution itself. The term was employed to describe th6 bubblirfg or boiling condition seen in the production of wine, prior to the time that yeasts were discovered. However, after Pasteur's discovery, the word became used with microbial activity, and later with enzyme activity. Currently the term is used even to describe the evolution of carbon dioxide gas during the action of living cells. Neither gas evolution or the presence of living cells is essential to fermentative action, however, as seen in lactic acid fermentations, where no gas is liberated, and in fermentations accomplished solely with enzymes.
There is a clear difference between fermentation and putrefaction. Fermentation is a decomposition action on carbohydrate materials; putrefaction relates to the general action of micro-organisms on proteinaceous materials. Fermentation processes usually do not evolve putrid odors, and carbon dioxide is usually produced. In putrefaction, the evolved materials may contain carbon dioxide, but the characteristic odors are hydrogen sulfide and sulfur containing protein decomposition products. A putrid fermentation is usually a contaminated fermentation. Putrid kreut or pickles result from microbial growths decomposing protein, rather than the normal fermentation of carbohydrates to acid.
INDUSTRIALLY IMPORTANT ORGANISMS IN FOOD PRESERVATION
There are three important characteristics micro-organisms should have if they are to be useful in fermentation and pickling.
- The micro-organisms must be able to grow rapidly in a suitable substrate and environment, and be easily cultivated in large quantity.
- The organism must have the ability to maintain physiological onstancy under the above conditions, and yield the essential enzymes easily and abundantly in order that the desired chemical changes can occur.
- The environmental conditions required for maximum growth and production should be comparatively simple.
The application of micro-organisms to food preservation practices must be such that a positive protection is available to control contamination.
The micro-organisms used in fermentations are notable in that they produce large amounts of enzymes. Bacteria, yeasts, and molds, being single cells, contain the functional capacities for growth, reproduction, digestion, assimilation, and repairs in a cell, that higher forms of life have distributed to tissues. Therefore it is to be anticipated that single celled complete living entities, such as yeasts, have a higher enzyme productivity and therefore fermentative capacity than found with other living creates (Table 7.2).
Enzymes are the reactive substances which control chemical reactions in fermentation. The micro-organisms of each genus and species are actually a warehouse of enzymes, with its own special capacity to produce and secrete enzymes. Man has yet to learn to synthesize them.
A dry gram of an organism endowed with high activity lactose fermenting enzymes is capable of breaking down 10,000 grams of lactose per hour. This great chemical activity is associated with the simple life-process requirements of the organisms, the ease with which they obtain energy for life, their great growth capacity and reproduction rate, and their great capacity for maintenance of the living entity. One generation may occur in a matter of minutes.
But there is balance in effort. In living, the organisms consume energy. The product of their actions is a substrate of lower energy than that native material upon which they were planted. But, the product of the activity in the instance of wine is one which man generally enjoys more than the native juice, from which the wine was produced.
ORDER OF FERMENTATION
Micro-organisms have available carbohydrates, proteins, minerals and minor nutrients in native food materials.
It appears that micro-organisms first attack carbohydrates, then proteins, then fats. There is an order of attack even with carbohydrates, first the sugars, then alcohols, then acids. Since the first requirement for mictobial activity is energy, it appears that the most available forms, in order of preference, are the CH2, CH, CHOH, and COOH carbon linkages. Some linkages such as CN radicals are useless to micro-organisms.
Types of Fermentations of Sugar
Micro-organisms are used to ferment sugar by complete oxidation, partial oxidation, alcoholic fermentation, lactic acid fermentation, butyric fermentation, and other minor fermentative actions.
a) Bacteria and molds are able to break down sugar (glucose) to carbon dioxide and water. Few yeasts can accomplish this action.
b) The most common fermentation is one in which a partial oxidation of sugar occurs. In this case, sugar may be converted to an acid. The acid finally may be oxidized to yield carbon dioxide and water, if permitted to occur. For example, some molds are used in the production of citric acid from sugar sulutions.
c) Yeasts are the most efficient converters of aldehydes to alcohols. Many species of bacteria, yeasts, and molds are able to yield alcohol. The yeasts, Saccharomyces ellipsoideus, is of great industrial importance in alcoholic fermentations.
The industrial yeasts yield alcohol in recoverable quantities. While other organisms are able to produce alcohol, it occurs in such mixtures of aldehydes, acids, and esters that recovery is difficult. The reaction from sugar to alcohol is many stepped.
d) Lactic add fermentations are of great importance in food preservation. The sugar in foodstuff may be converted to lactic acid and other end products and in such amounts that the environment is controlling over other organisms. Lactic acid fermentation is efficient, and the fermenting organisms rapid in growth. Natural inoculations are such that in a suitable environment, the lactic acid bacteria will dominate, i.e., souring of milk.
[e - -o - -jf
e) Butyric fermentations are less useful in food preservation than those above. The organisms are anaerobic, and impart undesirable flavors and odors to foods. The anaerobic organisms capable of infecting man causing disease are commonly butyric fermenters. Carbon dioxide, hydrogen, acetic acid, and alcohols are some of the other fermentation products.
f) In addition to the above three is a fermentation which involves much gas production. It is useful in food preservation, although gas production has disadvantages. Energy-wise it is less efficient to produce gases (carbon dioxide and hydrogen) which have little or no preserving power in concentrations found in comparison with lactic acid. Too, the important food spoilage organisms are capable of growing in such environments. In gassy fermentations, sugar molecules are altered to form acids, alcohols, and carbon dioxide.
It is usually necessary to include some other controlling influence such as adding sodium chloride to a substrate with this form of fermentation.
g) There are many fermentative actions possible in foods which act in detriment to the acceptability of treated foods. Generally the organisms capable of attacking higher carbohydrates such as cellulose, hemicelluloses, pectin, and starch will injure the texture, flavor, and quality of treated foods.
Fermentation Controls
Foods are contaminated naturally with micro-organisms, and will spoil if untended. The type of action which will develop is dependent upon the conditions which are imposed. The most favourable to a given type of fermentation under one condition will be altered by slight changes in a controlling factor. Untended meat will naturally mold and putrefy. If brine or salt is added, entirely different organisms will take over.
The pH Value of Food is a Controlling Factor. Most foods in native, fresh form which man consumes as food are acid. Vegetables range in pH value from 6.5 down to 4.6. Fruits range from 4.5 down to 3.0. Animal fresh when freshly killed is approximately neutral (7.2) but within two days the pH value will be approximately 6.0. Milk has a pH value near 6.4.
Inasmuch as the two important fermentations in such foods are oxidative and alcoholic, the growth of organisms will be controlled by the acidity of the medium. In fruits and fruit juices, yeasts and molds will quickly establish themselves. In meats, yeasts are less active than bacteria. In milk, an acid fermentation is established in the matter of a few hours.
Source of Energy. Inasmuch as the immediate need of micro-organisms is a source of energy, the soluble, readily available carbohydrates influence the microbial population that will dominate. In milk, the sugar is lactose; those organisms which quickly mount in numbers are the lactose fermenting organisms. Because suitable energy sources are generally available to micro-organisms in man's foods, energy sources are not usually a limiting factor, with certain exceptions (as milk).
Available of Oxygen. The degree of anaerobiosis is a principal factor controlling fermentations. With yeasts, when large amounts of oxygen are present, yeast cell production is promoted. If alcohol production is desired, a very limited oxygen supply is required (Fig. 7.1).
Molds are aerobes, and are controlled by its absence. Bacterial populations which will dominate a substrate may be manipulated by their oxygen requirements, and its availability.
The end product of a fermentation can be controlled in part by the oxygen tension of the substrate, other actors being optimum.
Temperature Requirements-Each group of microÂorganisms has an optimum temperature for growth; the temperature of a substrate therefore exerts a positive control on their growth. To obtain the maximum performance during fermentation, the optimum temperature for the organisms must be created. Examples are available in milk fermentation and also in acetic acid production.
Milk held at 32°F. has little microbial activity, and retarded expansions in bacterial numbers. At 40°F. there is slight growth of organisms, and more rapid development of off-flavours.
At 70°F. Streptococcus lactis growth is usually dominant. At 100°F. Loctobacillus bulgaricus populations commonly dominate in milk. At 150°F. most organisms will be killed, but Lactobacillus thermophilus can grow. At 160°F. souring of milk is generally demonstrated to be due to the presence of Bacillus calidolactis. The temperature of milk is seen to be a controlling factor in the growth of the above organisms, other factors being equal.
The acetic acid bacteria are temperature sensitive, having definite and peculiar temperature relations, important in vinegar making. At 45°F. the organism grows slowly, cells are short and usually broad. At 70°F. the cells appear normal, growing and developing into chains of cells of varying number. Cell walls become swollen. At 100°F. long thread-like transparent filaments with no visible cross walls and with irregular bulging, sometimes branching, have been observed. This condition appears to be temperature induced, physiologically malfunctioning, pathological condition. Interestingly, lowering the temperature of the substrate to 80°F. that used in the United States in vinegar production, results in the production of some cells with normal characteristics and behaviour.
The temperature at which a food is held will determine within certain limits the nature of the organisms capable of either yielding the desired fermentation, or spoilage, whichever the case may be.
The Action of Sodium Chloride in Conrolling Fermentations. Salt is one of the most important food adjuncts in food preservation. In drying it has been shown to have beneficial effects. In fermentations, salt can exert a role in sorting the organisms permitted to grow. The amount of salt added determines whether or not any organisms can grow, and what types will grow, which therefore controls the fermentation activity, other factors being equal (Table 7.3).
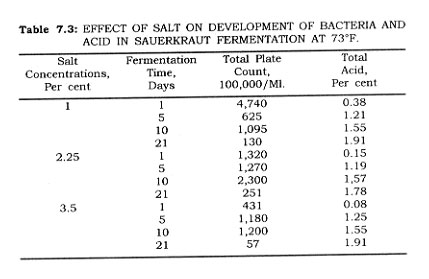
Salt in solution in food substrates exerts a growth repressing action on certain micro-organisms, can be made to limit moisture availability, can dehydrate protoplasm and cause plasmolysis.
In foods containing salt as a preservative, the salt has been ionized, collecting water molecules about each ion. The process is called ion hydration. The greater the concentration of salt, the more water employed to hydrate ions. A saturated salt solution at a temperature is one that has reached a point where no further energy is available to dissolve the salt. At this point (26.5 per cent sodium chloride solution at room temperature) bacteria, yeasts and molds are unable to grow. It has been postulated that there is no free water available for microbial growth.
In describing the preservative action of salt, one must consider the dehydration effect, the direct effect of the Cl ion, reduced oxygen tension, and interference with the action of enzymes.
Organisms can be selected or sorted on the basis of salt tolerance. This is a well employed procedure in identification of bacteria. Too, it is functional in controlling fermentations.
Certain lactic acid bacteria, yeasts, and molds either tolerate or adapt to moderate salt solutions. Sporeforming aerobes and anaerobes are not tolerant to salt solutions, or they are sufficiently inhibited that subsequent production of acid by lactic acid bacteria so supplements the inhibition influence of salt that sporeforming bacteria are of little concern, providing both conditions of salt and acid are operative.
Proteolytic bacteria and pectolytic organisms are also inhibited by salt and acid solutions. However, these organisms are more sensitive to acid than salt. In conditions where mold, tolerant to salt and a utilizer of acid, is permitted to grow, decreasing the acidity of the substrate, then putrefactive and pectolytic organisms can be anticipated to increase in numbers, and cause food spoilage.
Salt, vinegar, and spices are commonly used in complementary action in many fermented food products. Species vary in their antibacterial activity, some (mustard oil) being very active, others (black pepper) having little activity.
Sources of Salt
Salt is one of man's highly valued accessory materials, and has been used even to control men, i.e., the salt taxes in India. Nations attempt to become self-sufficient in salt production today, as they did five thousand years ago. Man's need for salt does not diminish.
There are three major sources of salt. Solar salt is obtained by evaporation of salt water, either from the oceans or from inland salt lakes. Mined salt, commonly referred to as rock salt, is obtained from mines, operating a thousand feet and more below the surface of the earth. Some salt is pumped from deeper subterranean salt deposits, using water as the transporting medium, and is called welled salt.
Salt obtained from solar distillation contains chemical impurities, and salt tolerant, halophilic micro-organisms. Mined and welled salts are generally free from these contaminating organisms.
Wine and Beer
Wine and Beer or similar fermented products originated in antiquity. Alcoholic beverages were discovered by man in many areas on earth, with the exception of the early American Indian. There was a fermented cactus juice known to certain tribes in the Southwest, but, evidently, alcohol was not a commodity in America as it was in the ancient Egyptian. Greek, Roman, and Oriental civilizations.
The ability to produce pleasant, palatable effervescent beverages by fermentation of natural fruit juices is a demonstration of man's inherent ingenuity. From earliest recorded history, wine and beer have been important items of trade.
In the United States the term wine means the product of an alcoholic fermentation of the juice of sound ripe grapes. Any other fruit juice wine must carry the notation, for example, that it is "cherry" or "back-berry" wine indicating the source of the fruit juice.
The formation of alcohol form sugar is accomplished by yeast enzymes, which are contributed by the growing yeasts. Saccharomyces ellipsoideus is the true wine yeast. There is normally a population of yeasts on grape skins. When a viable yeast cell has access to sugar from the juice of a crushed grape, fermentation begins. Usually the true wine yeast is present in sufficient quantities to dominate the fermentation. But, the juice may be heated to kill contaminating organisms, and reinoculated with pure culture wine yeasts. In certain special instances, i.e., effervescent sparkling wine, it is essential that a secondary fermentation occur, in bottle, to develop a carbon dioxide content.
In a suitable environment and substrate,- the-amount of alcohol produced depends upon the amount of sugar present and the efficiency of the yeast in converting the sugar to alcohol. Grapes for wine making vary in sugar content from 12 to 25 per cent. The ability of different yeast strains to survive in increasing alcohol concentrations varies. Eventually the yeast is unable to tolerate the fermentation products, usually when 12 to 16 per cent alcohol has been developed. Some yeasts can tolerate 18 per cent alcohol. Fortified wines are natural fermentation products to which alcohol has been added. For each 100 parts of sugar present in the juice, approximately 51 per cent alcohol and 49 per cent carbon dioxide are produced.
Dry wines are wines containing little unfermented sugar (Table 7.4).
Aging of wines improves the flavor and bouquet, due to oxidation and formation esters. These esters of higher acids formed during aging give the ultimate pleasing bouquet to well aged wine. Aged wine may be polished by filtration to give a clear, bright appearance prior to bottling.
Sulfur dioxide may be added to crushed grapes to retard the growth of contaminating organisms. Mustard seeds have-been used for centuries in Europe and the Near East as additives to crushed grapes for wine making. Recently it has been found that mustard seed contains an anti-bacterial agent, in the essential oil of mustard.
White wines do not have extracted red pigments from grape skins, and are lower in tannins and extracts.
Wine making remains an art and a science, and dependent on nature to yield environmental conditions for grape culturing. Certain very localized areas have the proper combination of soil type, sunlight, temperature, and rainfall required for grape growing, and these have become known throughout the societies of man. Examples are found in the Rhine and Rhone valleys of Europe, in areas of New York and California. Expert wine tasters are able to locate generally the vineyard area from which the grapes for a wine have come.
Beer and ale are fermentation products with characteristic flavor and aroma from malt and hops. The alcoholic content ranges from 3 to 7 per cent. The strain of yeast used, the composition of the wort, and the temperature of fermentation are controlling factors. Hops has at least two antibacterial components in addition to having a role in flavouring. Boiled wort must be inoculated with yeast at the start of the fermentation. Packaged beer is usually pasteurized, while barrelled beer is not. Pasteurization temperatures of 148°F. are commonly used.
Cider Vinegar-Vinegar is a condiment prepared from various sugary or starch}' materials by alcoholic and subsequent acetic fermentation. It consists principally of a dilute solution of acetic acid in water, but also consists principally of a dilute solution of acetic acid in water, but also contains flavoring, colouring and extracted substances, fruit acids, esters, and inorganic salts, varying according to its origin. Although acetic acid is the active ingredient of all vinegars, these additional substances give distinctive and pleasing quality to the product. Vinegar may be produced from the juices of most fruits such as apples, grapes, cherries, and pears, but cider vinegar has always been the most popular in the American home. The principles involved and method of preparing cider vinegar will be discussed herein.
The Food and Drug Administration of the United States defines cider vinegar or apply vinegar as the product of alcoholic and subsequent acetic acid fermentation of the juice of apples. According to regulations, the word vinegar. unqualified, can be applied only to vinegar made from apples.
Vinegar contains at least 4 gm. of acetic acid per 100 ml (1.0 fluid ounce per 24.1 fluid ounces). Dealers generally use the term "grain strength" rather than per cent acetic acid. Per cent acid times ten gives the strength of the vinegar in grains. Thus a vinegar containing four grams of acefic acid per 100 cubic centimeters (approximately four per cent acid) is referred to as 40 grain. Vinegar offered for sale, must contain at least this much acid, must be wholesome, made from sound edible fruit, and must be properly labeled.
PRINCIPLES AND PRESERVATION OF BAKERY PRODUCTS
INTRODUCTION
Cooking is the art of preparing foods by heating until they are changed in flavor, tenderness, appearance, and chemical composition. Baking is a form of cooking that is carried out in an oven, and the origin of the process is as old as recorded history. Baked bread is not only one of the most ancient of foods manufactured by man, it is also the food most widely eaten by man. Furthermore, there is no natural counterpart to bread in nature.
A century ago, more than 95 per cent of all bread consumed in the United States was baked at home; currently, more than 95 per cent of all the bread consumed here is manufactured outside the home. Today, some 50,000,000 loaves of bread are baked daily in the United States, and some factories can produce upwards of a million dollars worth of various barkery products a day. Baking must then have evolved into a highly sophisticated technology and the effective preservation of bakery products must be essential to it. In this chapter, the principles of baking and the technologies of preserving bakery products will be explored.
PRINCIPLES OF BAKING
The actual baking process is really the last and most important step in the production of bakery products. Through the agency of heat, an unpalatable dough mass is transformed into a light, porous, easily digestible and most appetizing product. The changes involved are complex and basic.
The biological activities that had been taking place in the dough are stopped by baking with the destruction of the microÂorganisms and enzymes present. The unstable colloidal system present is stabilized. The basic characteristics of the native starch and proteins are drastically changed. At the same time, new flavoring substances are formed, including caramelized sugars, pyrodextrins and melanoidins, which give the baked product its desirable and distinctive organoleptic properties.
All of the desirable reactions involved in changing a dough into a bakery product must occur in certain sequences and require controlled conditions. While each of the chemical and physical reactions that occur in a baking dough are still not fully understood, there is growing body of information on the subject, and it has expanded our insight into the baking process.
Because of the significance of this process, it is important that the present knowledge of it be explored, using bread production as the basic vehicle for the discussion. Its expansion to include other bakery products follows.
Dough
The protein of wheat flour is unique is that when the flour is mixed with water in certain proportions, the protein forms an elastic colloidal mass or dough which can hold gas and which will form a spongy structure when baked. It is this characteristic of wheat flour, not found in other cereal grains, which permits the manufacture of soft white bread.
Initially, the protein exists in a coiled condition in dough, and gives it its elastic behavior. The linkages between the chains are not equally strong at all points so that when the dough is worked or mixed, some of the linkages break while others remain intact. This slow but constant adjustment that occurs within the dough during mixing takes place in the presence of the starch and other materials present in the dough. (See Table 12.1). The starch-water paste itself has distinct physical characteristics, and these also come into play from a physical point of view. Salt also influences the structure, as do sugar, shortening, emulsifiere, enzymes, etc.
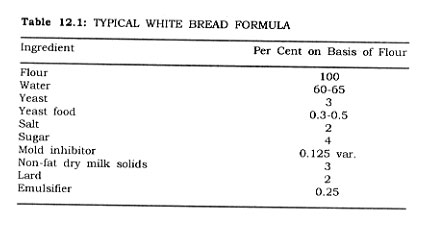
When the flour is first wet with water, the protein present is found to be in a random distribution. As mixing occurs, the protein chains become oriented into a somewhat parallel orientation or position. As this condition is reached, the dough changes in appearance, and takes on a smoothness characteristic of an adequately mixed dough; furthermore, the protein now has its maximum elasticity. Mixing beyond this point causes more breakage of molecular linkages, and the dough becomes soft and adhesive in character.
Different kinds of wheat flours require different amounts of mixing to reach a satisfactory smoothness. This is explained by Wall (1967) as due in part to the variable rates of hydration of their proteins (mainly gluten). There are also differences in wheat varieties in terms of the configuration of subtle molecular structures and the various ways in which they are bound together in their gluten strands.
Doughs contain gas cells. Baker (1941) separated some of these intact gas cells and found that the cell material itself was glutinous and contained about 45 per cent gluten and 25 per cent starch on a dry basis. Observing gas cell formation, and enlargement during fermentation of doughs, he concluded that starch was not necessary for gas cell formation. The gas nucleus which forms the bubble in the dough aparently originates in the glutinuous mass. As the bubble expands, the amount of gluten needed to satisfy the surface needs of the growing bubble is drawn from the hydrated starch-gluten matrix of the endosperm material in the dough.
The source of the gas was found to be dissolved gases which diffuse into cells formed from air being beaten into the dough during mixing. Baker further noted that, surprisingly, yeasts themselves were incapable of originating gas cells in doughs. This also suggests why rapid mixing is most important in cake batter, in order to incorporate as many minute air cells or bubbles as possible, which later, during baking, will expand and form the fine texture desired in baked cakes. The gas needed for dough expansion during baking of course can come from other sources, such as the added chemical leavening agents, from steam generated during baking, and from expansion of the occluded air bubbles in the batter during baking. The structure will then be set, hopefully, at the maximum expansion during baking, yielding the desired texture and volume.
Influence of Flour Proteins
As noted above, the proteins of wheat flour contribute in a most significant manner to the properties of doughs and to their baking performance. There have been many investigations of the proteins in flour, and it is well agreed that albumin, globulin, gliadin and glutenin are present. Gliadin and glutenin are by far the major fractions, and constitute most of the gluten ball obtained when dough is washed free of starch.
An excellent review of present knowledge concerning the origin and behavior of flour proteins was prepared by Wall (1967). In general, it is found that gluten is not uniformly distributed throughout the endosperm of the developing wheat grain, but concentrated in protein bodies. These seem to be held by a network of lipid containing proteins. (Proteins outside the protein bodies are predominantly albumin and globulin.) It is thought that these bodies serve as centers for synthesis of gliadin and glutenin, which eventually would be the protein reserves for the germinating seed.
As the wheat grain matures, starch granules and protein bodies grow. When the grain dries, Wall has found that these endosperm components become tightly packed. The matrix protein and protein bodies then are not readily distinguishable, and they form a rather continuous network binding together the starch granules.
When the grain is milled, the endosperm is broken, selectively separating the protein network and the starch granules. Maturing of wheat by aging appears to cause oxidation of sulfhydryl and other reducing groups in the endosperm. Moisture tempering of the grain prior to milling favors better protein release from starch granules during milling.
During dough formation, there are many factors influencing the protein. In the hydration stage, water penetrates the protein structures and overcomes some of the forces tending to cause the molecules to adhere to each other.
In mixing, there is an orientation of protein structures as noted earlier. The protein structures are changed from their native condition in bodies to films which form a continuous network containing the starch granules.
During proofing and baking, the network is first stretched then denatured. However, some of the native proteins apparently can still be recovered from baked bread (Wall 1967).
Flour Improvers
Bakers learn by experience that aged flour perfoms better in baking than freshly milled or "green" flour, and that a superior product is also produced. Today, according to Tsen and Hlynka (1967) small amounts of oxidizing agents are added to flour to accomplish the maturing process. Such oxidizing agents are called flour improves. The commonly used agents are shown in Table 12.2. All such flour improvers are known as sulfhydryl oxidizing agents. The situation is reviewed in depth by Tsen and Hlynka (1967).
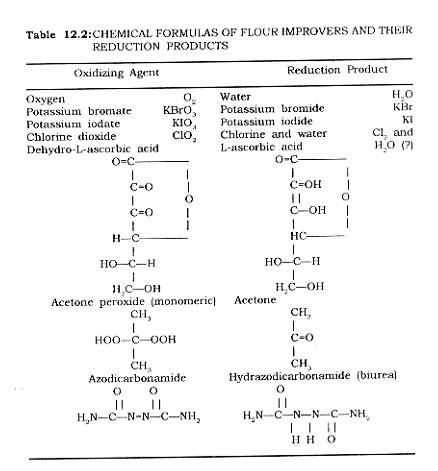
Other Components of Flour
Wheat flour contains about 0.8 per cent free lipids and about 1.0 per cent bound lipids, according to typical analyses. Pomeranz (1967) has reviewed the importance of lipids in baking. He found that flour lipids improve low strength flours, improve dough development and improve the retention of freshness in baked products.
Wheat flour contains about 0.5 to 0.8 per cent of water soluble pentosans. They have the property of dissolving in water to produce very viscous solutions. Neukom^and co-workers (1967) reported that a 0.5 per cent solution is so viscous that it will not pour and a four per cent solution forms a soft gel. When dough is formed, water is taken up by components in proportion to their ability to hydrate. It has been calculated that about 46 per cent of the water in dough is associated with the starch, about 31 per cent with gluten and about 23 per cent with the pentosans. This is a surprising finding since the soluble pentosans are present in such small amounts. While their influence on baking qualities is being explored, the insoluble pentosans are also being looked at for their depressing effects on quality in bakery products (Reed 1967).
Yeast Raised Dough Products
Yeast raised bakery products are made generally by either a straight dough method or a sponge and dough method. Each has advantages and limitations.
Straight Dough Method-This is a single step process in which all the ingredients are mixed together in a single batch. Mixing in this case is conducted until the dough mass achieves the desired smoothness and appearance, and develops necessary elasticity. The temperature of the dough as it comes from mixing should be between 78° and 82°F. (Pyler 1967). Temperatures higher than these are generally undesirable unless fermentation times are being reduced, in which case more yeast is used.
The advantages of this method are: labor requirements are at a minimum; fermentation times are less than with sponge and dough methods, hence fermentation losses are reduced; and, the straight dough method is reported to generate better flavor in the baked products.
The disadvantages of the method are: inflexibility, requiring fixed fermentation times; ripe doughs must be baked when ready; and little can be done to correct for over-fermentation.
Sponge and Dough Method-This consists of two different steps: one, formation of the sponge, and two, development of the dough. The sponge stage involves the mixing of part of the dough ingredients followed by a preliminary fermentation. In the dough stage, the fermented sponge is combined with the remaining ingredients, mixed, and allowed to ferment a second time of short duration.
The sponge generally contains about 50 to 75 per cent of the total flour to be used, the total yeast and yeast foods, the malt and sufficient water to yield a slightly stiff dough. The remaining ingredients are added in the second step. Adjustments in mixing procedures are possible to correct for flour defects.
The mixing procedure is about that of the straight dough method, except that temperatures are held lower at about 72° to 78°F.
The advantages of this system are: there is a saving of about a fifth of the yeast needed; bread baked by this process tends to have greater volume and improved texture, and the method is flexible, permitting sponges to be held longer, without losses in quality.
Obviously, the disadvantage of more labor and processing costs, plus more fermentation and evaporation losses, are to be acknowledged.
Heat Generated During Mixing Doughs
Dough mixing is accompanied by a measurable rise in the temperature of the dough mass. The sources of heat are mainly from the energy released by hydration of the flour and the heat produced by friction during mechanical mixing of the dough.
The heat produced must be removed by one means or another if dough temperatures are to be maintained at appropriate levels. The temperature control needed is generally brought about by using crushed ice, using cooled ingredient water, or by the application of refrigeration to the mixing bowl.
Heat of Hydration
There is heat evolved by most substances when they take up water. The amount of heat varies with the amounf of water absorbed, which depends upon the nature of the absorbing material. A completely dry substance evolves the greatest amount of heat on hydration because a greater proportion of water molecules will be firmly bound to its surfaces. As the moisture content of the substance increases, less and less heat is liberated. In substances such as flour the initial moisture content will have a marked influence on the heat of hydration. For example, flours with moisture contents of 13.1 and 8.7 per cent produced finished doughs whose temperature differed by about 6°F. Lincoln, Dirks and Harrel have tabulated the relationship between moisture content and amount of heat of hydration for wheat flour. Table 12.3 was adapted from their data by Pyler (1952).
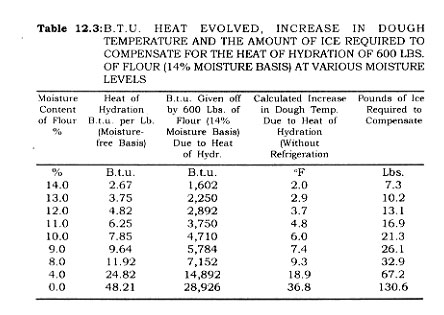
Cooling Requirements
The capacity of refrigeration needed for a given production volume may be calculated. As was noted above, the heat load involved in dough mixing includes the sensible heat of ingredients, the heat of hydration of flour, and the heat put in by the mixer as friction heat. To these could be added a fourth factor, the heat absorbed by mixer jacket from the environment.
In order to calculate the B.t.u.'s contributed by each of these sources, certain factors must be known. These include the sensible heat of ingredients, the specific heats of flour, dough and water, the heat of hydration of flour at different moisture contents, the heat generated by the motors, etc. The generally used values for these factors reported by Pyler (1967) are as follows:
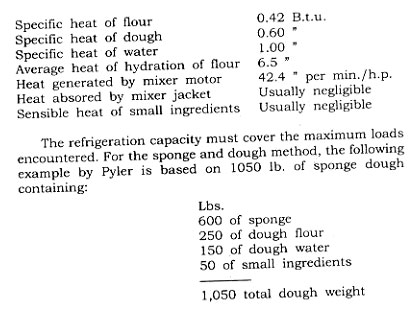
He added the following assumptions: the mixing schedule requires 3 mixes per hour with a mixing time of 10 minutes per dough, and a final dough temperature of 78°F; the flour enters the mixer at 90°F. and has an average moisture content corresponding to a heat of hydration of 5.6 B.t.u.'s per pound; ingredient water is chilled to 40°F. and the sponge is returned at 83°F; and the mixer uses 25 h.p.
The capacity of a direct expansion jacket cooler required under these conditions may be calculated as follows:
To cool the sponge from 83°F. to 78°F., a difference of 5°F., requires the withdrawal of B.t.u.'s equivalent to the sum of the weight of the sponge multiplied by the specific heat of dough multiplied by the temperature difference between 83° and 78°, or 600 x Q.6 * 5 = 1800 B.t.u.
The cooling requirement to reduce the flour from 90°F to 78°F. is calculated by the same procedure, namely, by multiplying the weight of the flour by its specific heat and by the temperature difference, or 250 x 0.42 x 12 = 1260 B.t.u.
To remove the heat of hydration of the flour requires the withdrawal of B.t.u.'s equivalent to the sum obtained by multiplying the weight of the flour by the hydration factor, or 250 x 6.5 = 1625 B.t.u.
In this example Pyler considered the cooling of the 50 Ib. of small ingredients, part of which reach the dough at 78°F. or lower, as such a minor item that it may be ignored in the overall calculation.
The heat load generated by a 25 h.p. mixer motor running 10 minutes during each mix is equal to the mixer factor per horsepower per minute multiplied by 10, or 42.4 x 25 x 10 = 10,600 B.t.u
.
The heat absorbed by a well-insulated mixer jacket from the environment is usually small compared to the above.
Chilled ingredient water at 40°F. will withdraw B.t.u. from the rest of the dough mass. The amount of this cooling effect is equal to the weight of the water multiplied by its specific heat multiplied by the temperature difference between 40°F. and 78°F., or 150 x 1 x 33 = 5,700 B.t.u.
Pyler summarized the heat load of a 1050 Ib. dough under the assumed conditions, as follows:
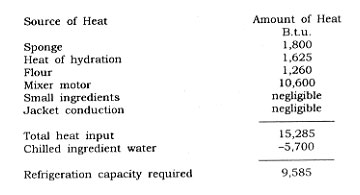
The refrigeration unit must absorb a heat load of 9,585 B.t.u. in 10 minutes of mixing time or at a rate of 958 B.t.u. per minute. Since refrigeration units are rated on an hourly capacity, a machine capable of absorbing 958 x 60, or 57,480 B.t.u. per hour is required for the above heat loads, or a unit of approximately 10 h.p.
A full and broad ranging discussion of the subject is presented by Matz (1960) and Pyler (1952).
Continuous Bread Making Process
Continuous bread making systems are designed to bring together the necessary ingredients, mix them, develop a dough and bring it to a condition for baking. The ingredients are thoroughly mixed into a homogenous condition, passed to a dough pump, and metered into a developing apparatus. In the developer, the dough is kneaded to bring about the desired structure and gas holding capabilities.
The developed dough is then extruded, shaped, and deposited in proper weights into pans on a moving belt. The movement of the pans is such that they are synchronized with the above. The proper amount of developed dough is deposited into a pan, which moves by belt to a proofing zone.
There are two major types of continuous dough making systems currently in wide use: the Do-Maker and the AmFlow. A full description of each has been presented by Fortmann (1967).
Typical Formulations for Yeast Raised Bakery Products Up to this point, we have focused mainly on "bread. In practice, the only difference between various yeast raised bakery products is their richness, or the amounts of sugar, shortening, milk solids and egg solids used. A comparison of some general formulations is shown in Table 102.
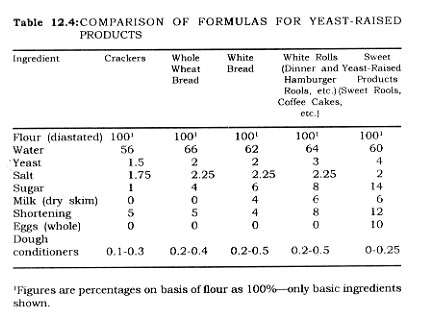
Ingredients available for baking continue to improve and new ingredients are constantly coming to the fore. An excellent review of baking ingredients is presented in reference: the use of enzyme supplements in baking (Reed 1967); the present situation and future potentials of baker's yeasts, (Schuldt 1967); a review of emulsifiers and their uses in baking (Endres 1967); developments in egg solids and in the egg industry (Kahlenburg 1967); shortenings, (Koren 1967); milk and dairy products (Mertens and Mykleby 1967); and, flour as an ingredient (Pyler 1967).
Baking Schedules
As noted earlier, baking itself is perhaps the most critical aspect of the whole series of events leading to high quality baked products. Typical baking temperatures and times are shown in Table 12.5. Also included in this table are the internal time-temperature relationships during the baking of various types of bakery products.

However, of more concern is the baking reaction itself, since insight into it is deemed most important.