Biodegradable Plastics - Developments and Environmental Impacts
INTRODUCTION TO BIODEGRADABLE PLASTICS
The 'biodegradability' of plastics is dependent on the chemical structure of the material and on the constitution of the final product, not just on the raw materials used for its production. Therefore, biodegradable plastics can be based on natural or synthetic resins. Natural biodegradable plastics are based primarily on renewable resources (such as starch) and can be either naturally produced or synthesised from renewable resources. Non-renewable synthetic biodegradable plastics are petroleum-based. As any marketable plastic product must meet the performance requirements of its intended function, many natural biodegradable plastics are blended with synthetic polymers to produce plastics which meet these functional requirements.
Many polymers that are claimed to be 'biodegradable' are in fact 'bioerodable', 'hydro-biodegradable' or 'photo-biodegradable'. These different polymer classes all come under the broader category of 'environmentally degradable polymers'. For the purpose of this document the term 'biodegradable plastics' shall imply 'environmentally degradable plastics'. The classes of biodegradable plastics considered, in terms of the degradation mechanism, are:
- Biodegradable
- Compostable
- Hydro-biodegradable
- Photo-biodegradable
- Bioerodable
These definitions of degradation are used throughout the report to describe the degradation processes of the 'biodegradable plastics' currently available or under development. Definitions of these degradant mechanisms for different materials are provided below.
Biodegradable
The failure of early 'biodegradable' plastics to properly degrade led to the American Society of Testing and Materials (ASTM) creating definitions on what constitutes 'biodegradability'. The ASTM definition, updated in 1994 (ASTM Standard D-5488-84d), has led to the establishment of labelling terminology for packaging materials.
The ASTM defines 'biodegradable' as:
"capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, or biomass in which the predominant mechanism is the enzymatic action of microorganisms, that can be measured by standardized tests, in a specified period of time, reflecting available disposal condition."
Biodegradation is degradation caused by biological activity, particularly by enzyme action leading to significant changes in the materials chemical structure. In essence, biodegradable plastics should break down cleanly, in a defined time period, to simple molecules found in the environment such as carbon dioxide and water.
Biodegradation rates are highly dependent on the thickness and geometry of the fabricated articles. While rapid breakdown rates are often quoted, these generally apply to thin films. Thick-walled articles such as plates, food trays and cutlery can take up to a year to biologically degrade.
Compostable
Compostable biodegradable plastics must be demonstrated to biodegrade and disintegrate in a compost system during the composting process (typically around 12 weeks at temperatures over 50°C). The compost must meet quality criteria such as heavy metal content, ecotoxicity, and no obvious distinguishable residues caused by the breakdown of the polymers. Compostable plastics are a subset of biodegradable plastics.
'Compostable' is defined by the ASTM as
"capable of undergoing biological decomposition in a compost site as part of an available program, such that the plastic is not visually distinguishable and breaks down to carbon dioxide, water, inorganic compounds, and biomass, at a rate consistent with known compostable materials (e.g. cellulose)."
Hydro-biodegradable and Photo-biodegradable
Hydro-biodegradable and photo-biodegradabe polymers are broken down in a two-step process -an initial hydrolysis or photo-degradation stage, followed by further biodegradation. Single degradation phase 'water-soluble' and 'photodegradable' polymers also exist.
Bio-erodable
Many polymers that are claimed to be 'biodegradable' are in fact 'bioerodable' and degrade without the action of micro-organisms - at least initially. This is also known as abiotic disintegration, and may include processes such as dissolution in water, 'oxidative embrittlement' (heat ageing) or 'photolytic embrittlement' (UV ageing).
Sections 3, 4 and 5 outline the current biodegradable polymer types, technologies and developments. Many blends of these materials, particularly starch and polyester blends, are also considered. Biodegradable plastics that are intended to be 'degradable in vivo' (in the body, i.e. implants), are considered to be beyond the scope of this study.
BIODEGRADABLE STARCH-BASED POLYMERS
Starch is a linear polymer (polysachcaride) made up of repeating glucose groups linked by glucosidic linkages in the 1-4 carbon positions. The length of the starch chains will vary with plant source but in general the average length is between 500 and 2 000 glucose units. There are two major molecules in starch - amylose and amylopectin. The alpha linkage of amylose starch allows it to be flexible and digestible.
Starch-based biodegradable plastics may have starch contents ranging from 10% to greater than 90%. Starch based polymers can be based on crops such as corn (maize), wheat or potatoes. Starch content needs to exceed 60% before significant material breakdown occurs. As the starch content is increased, the polymer composites become more biodegradable and leave less recalcitrant residues. Often, starch-based polymers are blended with high-performance polymers (e.g. aliphatic polyesters and polyvinyl alcohols) to achieve the necessary performance properties for different applications.
Biodegradation of starch based polymers is a result of enzymatic attack at the glucosidic linkages between the sugar groups leading to a reduction in chain length and the splitting off of sugar units (monosaccharides, disaccharides and oligosaccharides) that are readily utilised in biochemical pathways.
At lower starch contents (less than 60%) the starch particles act as weak links in the plastic matrix and are sites for biological attack. This allows the polymer matrix to disintegrate into small fragments, but not for the entire polymer structure to actually bio-degrade.
There are several categories of biodegradable starch-based polymers including:
- Thermoplastic starch products;
- Starch synthetic aliphatic polyester blends;
- Starch PBS/PBSA polyester blends; and
- Starch PVOH Blends.
Thermoplastic Starch Products
Thermoplastic starch biodegradable plastics (TPS) have a starch (amylose) content greater than 70% and are based on gelatinised vegetable starch, and with the use of specific plasticising solvents, can produce thermoplastic materials with good performance properties and inherent biodegradability. Starch is typically plasticised, destructured, and/or blended with other materials to form useful mechanical properties. Importantly, such TPS compounds can be processed on existing plastics fabrication equipment.
High starch content plastics are highly hydrophilic and readily disintegrate on contact with water. This can be overcome through blending, as the starch has free hydroxyl groups which readily undergo a number of reactions such as acetylation, esterification and etherification.
Developments
The CRC for International Food Manufacture and Packaging Science, Australia has developed its own version of TPS biodegradable plastics. These natural vegetable starch polymers have a amylose content greater than 70%.
Trials have been successfully performed using maize starch polymers as mulch film, and the material was found to perform as well as polyethylene film, with the added advantage that after harvest, the film can be simply ploughed into the soil. These natural starch polymers are now being commercialised through a new company called Plantic Technologies Ltd. based in Melbourne.
Applications
The applications of thermoplastic starch polymers are generally film, such as shopping bags, bread bags, bait bags, over wrap, 'flushable' sanitary product packing material, and mulch film.
Foam loose fill packaging and injected moulded products such as take-away containers are also potential applications. Foamed polystyrene can be substituted by starch foams that are readily biodegradable in some loose-fill packaging and foam tray applications.
Foamed starch loose-fills are rather easy products to produce and this area has become an early market for biodegradable plastics. During its preparation, raw starch is premixed with 25 to 50 weight percent water and fed into an extruder capable of imparting intensive shear and operating at high temperature (higher than the boiling point of water, i.e., 150-180°C). Under these conditions of shear and temperature, starch breaks down, loses its crystallinity, and gets plasticised with water, resulting in a homogenous amorphous mass. When this gelatinised starch/water mixture exits the extruder, the water that is present in the mass at a temperature higher than its boiling point expands into steam due to a sudden drop in pressure, and the foam is formed. Generally a plasticiser (such as glycerol) and another polymer (such as polyvinyl alcohol) impart more reproducible properties to starch foam.
Degradation Mechanisms and Properties
Along with the biodegradation of the polymers by sugar molecules, certain TPS grades are also fully water soluble.
Starch Synthetic Aliphatic Polyester Blends
Blends of biodegradable synthetic aliphatic polyesters and starch are often used to produce high-quality sheets and films for packaging by flat-film extrusion using chill-roll casting or by blown film methods since it is difficult to cast films from 100% starch in a melted state. Approximately 50% of the synthetic polyester (at approximately $4.00/kg) can be replaced with natural polymers such as starch (at approximately $1.50/kg), leading to a significant reduction in cost. Furthermore, the polyesters can be modified by incorporating a functional group capable of reacting with natural starch polymers.
Developments
Lim et al. studied the properties of an aliphatic polyester blended with wheat starch. The polyester was synthesized from the poly-condensation of 1,4-butanediol and a mixture of adipic and succinic acids. The wheat starch-polyester blends were found to have melting points near that of the polyester alone. A plasticiser was added to the starch, making the blends more flexible and processable than the polyester itself. Plasticised blends were found to retain a high tensile strength and elongation at the break point, even at high concentrations of starch.
Blending starch with degradable synthetic aliphatic polyesters such as PLA and PCL has recently become a focus of biodegradable plastic development. Biodegradable plastics can be prepared by blending up to 45% starch with degradable PCL . This new material is not strong enough for most applications, as the melting temperature is only 60°C and it gets soft at temperatures above 40°C. These drawbacks greatly limit the applications of the starch-PCL blends. Table 1 details some starch-PCL polymers that are commercially available.
Table l
PCL Polymers (Commercially Available)
| Polymer Type | Trade-name | Supplier | Origin |
| Starch-polycaprolactone (PCL) blends |
Mater-Biâ„¢ |
Novamont |
Italy |
| |
Bioflexâ„¢ |
Biotech |
Germany |
Applications
The applications for starch-synthetic aliphatic polyester blends include high-quality sheets and films for packaging and other film applications.
Several starch-based plastics are currently available on the Australian market. One of these is the 'BioBag', which is produced from the Novamont resin that has been around since 1994, and is made from corn starches in combination with fully biodegradable plastics or polylactic acid.
Recent Developments in the Biopolymer Industry
INTRODUCTION
The conventional definition of biopolymers, as defined by biochemists, is that they are biological macromolecules in which many identical or similar small molecules are covalently linked to one another to form a long chain. As indicated by Mohanty et al., biopolymers were not developed in nature with the intention of them serving as plastic materials. Rather, they were intended to act as cellular components which imparted the ability to survive in a given environment to an organism. However, as Mohanty et al. went on to explain, from a more progressive standpoint, biopolymers may be defined as products which are based on renewable agricultural or biomass feedstock, capable of behaving like conventional plastics in production and utilization, but degradable through microbial processes upon disposal. It is this progressive development of biopolymers which has led to a surging interest of a plastic and composite industry based on biological materials.
The development of biopolymer materials into widely accepted commercial products is being fueled by growing environmental consciousness of governments and citizens alike. As mentioned by Gerngross and Slater, worldwide production of plastics consumes approximately 270 million metric tons of fossil fuel each year, as a source of feedstock and energy. Although fossil fuels are still readily available, biopolymer researchers are in tune with the fact that a replacement feedstock for plastic materials will be required within a relatively short time. The development of bio-based products is a promising option in reducing this widespread dependence on fossil fuels. In addition, non-degradable conventional plastics such as polyethylene and polypropylene are filling landfills, at a time when space for waste disposal is at a premium in many regions of the world. Along this line, European governments have created the European Packaging and Landfill Directive, which restricts what volume of selected materials are permitted in landfills, thus driving consumers toward environmentally friendly (biopolymer) materials. In addition to reducing fossil fuel dependence and the rate of landfill use, biopolymers are also an excellent gateway to expanding value-added processing of agricultural materials. Numerous products have been developed which incorporate natural fibres (such as hemp, jute, flax, etc.) as reinforcements for polymer materials. In another aspect of value-added processing, biopolymers which are produced within plant material have also been developed. An example of this is a genetically modified corn developed by Monsanto where the seeds are harvested by conventional means, followed by polymer extraction from the stover.
There are also economic grounds for further development of the biopolymer industry. Returning to issues surrounding fossil fuels, the prices for crude oil have hit an all time high in recent months. Economically, plastic manufacturers are aware of the need to develop new methods to produce their product. Agricultural producers are also interested in exploring and developing the natural fibre processing industry, as it could potentially provide income from biomass, e.g. flax straw, which is usually burnt. Research at the University of Saskatchewan in the area of flax fibre utilization, and their development of a product known as FlaxticTM is a step toward expanding this industry in the Saskatchewan economy.
Work on further development of biopolymers is occurring in various regions around the world. In Canada, biopolymer research is primarily focused on fibre utilization, particularly in the provinces of Alberta, Saskatchewan, and Manitoba, where sustainability of the agricultural economy is crucial. At the University of Delaware, the ACRES (affordable composites from renewable resources) group has completed substantial research into the use of natural fibres for structural purposes, and the development of soy-based resins. Outside of North America, biopolymer research is also a focus of many institutions, particularly in Europe and Japan, where environmental consciousness is a legal requirement. In the United Kingdom, a partnership between twelve institutions has been formed, where researchers have worked together to understand polymer interactions. The information that they have gathered regarding the interactions between polymer bonds will prove to be quite useful in the development of new, innovative biopolymer materials. As reported by Mathieson, NEC, a Japanese company has successfully developed what they believe to be the first bioplastic appropriate for use in electronics packaging. The material is based on polylactic acid (PLA) enhanced with kenaf fibres, and boasts of a high thermal deformation temperature and bending modulus.
The intention of this work is to provide researchers with an update on the progress in the development of biopolymer materials. It is also intended to educate individuals who are not involved with biopolymers of the potential effect they will eventually have on the conventional plastic industry. The focus will be on materials or processes under recent development. Particular attention is warranted for fibre-reinforced composites, starch based materials, plant produced polymers, microbially produced polymers, and biologically based resins, coatings and adhesives. The future direction for biopolymer research will also be explored.
FIBRE-REINFORCED COMPOSITES
Natural fibres, such as those produced by hemp, flax, sisal, and jute are emerging as replacements for the glass fibre reinforcements usually found in a conventional polymer matrix. As explained by Van de Velde and Kiekens, the ecological and mechanical properties of the natural fibres are responsible for this opportunity. When natural fibre is incorporated into a polymer matrix, whether the matrix is based on natural or synthetic polymers, the final product is known as a biocomposite. Although the majority of composite materials include a plastic matrix and reinforcing fibre material, alternative matrix materials, such as concrete may also be used
Natural fibre-reinforced biocomposite materials have environmental and economic advantages over conventional composite materials. Glass fibres which are usually used to reinforce composite materials are formed through an energy intensive process detrimental to the environment. Natural fibres require decortication prior to use in composites, but much of this process can be completed in the field, or with chemical treatments, reducing the need for fossil fuel use as energy. In addition, particularly in the case of flax fibres, the biomass from which they are gathered has traditionally been burnt by producers, as a means of removing it from their fields. Therefore, the incorporation of it into biocomposites will provide an environmentally friendly way of disposing of the biomass.
The use of natural fibres as a matrix reinforcement is not a new concept. Early industrialists, such as Henry Ford were aware of the utility of materials such as hemp, as he constructed car components out of resin stiffened with hemp fibres. This vision, which was shared by American industrialists, scientists, and farmers in the 1920's and 30's, including Henry Ford, became known as "chemurgy". These liberal thinkers recognized that there is an enormous potential market for crops in industrial applications, given proper development of the technology. While this technology was left relatively unexplored during the industrious decades following World War II, an increasing number of researchers have begun working with biofibres since the 1990's.
Fibre incorporation in biocomposites is being researched in various regions around the world, particularly where agricultural production of natural fibres for industrial use is a viable option. The majority of the research is intended to find ways in which the fibres may be modified to have a stronger bond with the matrix (by surface modification), to change properties of the fibres to make them more suitable for industrial applications, or to refine the processes by which the fibres are removed from the plant stalk (or shives). At the University of Saskatchewan, chemical treatments intended to modify the surface of flax fibres in order to reduce their water absorption capabilities, and to increase their wettability and adhesion within a polymer matrix have been developed. The University of Delaware ACRES group has developed a novel process by which resins are applied to fibre mats. This process is known as vacuum-assisted resin transfer molding (VARTM), and has been used to manufacture bio-based composites from natural fibres in high volume application. In Europe, development of decortication techniques to remove the fibre from the shive of plants has been a focus for various researchers. An Italian team of scientists have refined the steam explosion process for removal of cellulose fibres from lignin and hemicellulose components. The chemical and surface modifications on the fibres from the steam explosion process greatly enhance their performance in composite materials.
The incorporation of natural fibres as reinforcements in composite materials is an exciting opportunity for those interested in value-added processing of agricultural materials. Continuing to work toward minimizing the cost of decortication and surface modifications of the fibres, and educating plastic manufacturers as to the benefits of natural fibre use are crucial in the development of the fibre reinforced biocomposite industry.
STARCH BASED MATERIALS
Starch is a pure, natural biopolymer, found in the roots, seeds, and stems of plants such as corn, wheat, and potatoes. It is suitable for chemical modification into a thermoplastic material, available for use in a variety of applications. As starch is fully biodegradable and easily renewable, it will continue to be an important component of the biopolymer industry.
The premise behind inclusion of starch in biocomposite materials is that upon disposal, the starch molecules will be consumed by microbes, and thus reduce the volume of the disposed article. Adding starch to a polymer mix reduces the volume of polymer (synthetic or natural) required for a given product, correspondingly reducing material costs. In addition, starch is a renewable resource, in that its use in plastic materials will minimize environmental damage caused by their manufacture. Unfortunately, the mechanical properties of natural starch are not ideal for its immediate and individual use in materials. Therefore, development of processing methods and composites incorporating starch are being researched by a number of organizations.
Research on starch-based materials has been widespread in recent years. In most cases, research has been intended to improve the mechanical properties of such materials. A number of American companies produce loose-fill packaging based on starch, and researchers have worked to improve their functional properties by incorporating biodegradable polymers as mechanical property enforcements. Results from Fang and Hanna's 2000 study indicated that a 25% addition of the biopolymer PLA greatly enhanced the mechanical properties of the foam. In a similar study, Ke and Sun blended starch and PLA to form a disposable and biodegradable plastic. However, the brittle nature of this material was seen as a drawback for its commercial use. In this regard, Ke and Sun added various plasticizers to the material, which improved the processability, flexibility, and stretchability of the material, by reducing intramolecular forces in the material. Researchers in The Netherlands have develolped a method by which polystyrene is grafted onto dissolved starch, using a twin screw extruder. The primary application for this product is as filler for thermoplastics. In Europe, the industrial production volume of starch is almost 7 million tonnes/year. Of this volume, approximately 50% of the starch is used for non-food applications, and approximately 30% of the starch is precipitated from industrial aqueous solutions. Enzymatic hydrolysis of potato processing waste has been studied by researchers at McGill University in Quebec, as a possible source of feedstock for fermentation to microbially produce a biopolymer.
Pure starch is fully degradable, under regular composting conditions. However, the decomposition temperature of starch is low, that it cannot be melted (and thus processed into usable materials) without being degraded. To enhance its processability, starch is combined with natural or synthetic polymers, often at the expense of its full biodegradability. Research into the rate of biodegradation of starch-based biopolymers in specific environments has been completed. Gilmore et al. studied the biodegradability of bioplastics in municipal leaf compost. The materials under consideration in their study included blends of starch with polypropylene (PP), polyethylene (PE), polycaprolactone (PCL), or poly-hydroxybutyrate-co-hydroxyvalerate (PHB/V). The research team found that when starch was combined with PP, PE, or PCL, very little change in the specimens was observed after a six month period of exposure to the leaf compost. This indicates that if synthetic polymers are combined with starch to produce a biopolymer, the biodegradability of the starch is overridden by the longevity of the synthetic polymer. In contrast, the starch-PHB/V samples were found to have lost approximately 60% of their mass after the six month period of exposure to leaf compost. In another study, starch-PHB/V composites were exposed to tropical coastal waters, and allowed to degrade for a twelve month period. The findings indicated that although both components degraded in the given environment, their half lives were quite different. In a 30/70 blend of starch/PHB/V exposed to coastal tropical waters, half of the starch had degraded after a 19 day period, while the PHB/V required 158 days to reach a comparable level of degradation. They concluded that blends with comparatively higher rates of starch content had faster rates of degradation.
As starch is a widely available and renewable agricultural product, the challenge for researchers is to incorporate its use into commercial materials. The greatest barrier to this utilization scheme is the imbalance between improving the mechanical properties of the starch-based biopolymers, while maintaining the desirable biodegradability of the end product.
PLANT PRODUCED POLYMERS
Some of the most recent advances in biopolymer research have focused on the genetic engineering of conventional plants, in order to develop molecular processes by which the plants actually produce usable polymer materials within their cellular tissue. The polymer material which researchers are interested in incorporating in plant tissues is polyhydroxybutyrate (PHB), which has mechanical properties similar to those found in polypropylene (PP). This fully biodegradable thermoplastic polyester material is commonly found in nature, as intracellular deposits in bacteria, formed as a carbon and energy storage mechanism when the bacteria is under stress, as explained by Geoffrey Coates of Cornell University. There is a conventional method of PHB production, which involves an energy-intensive and expensive process of sugar fermentation. However, select researchers have effectively found methods by which the genes required for PHB production can be engineered into plants which are already commonly produced by crop producers.
Corn, sugarcane, switchgrass, mustard, and alfalfa have all been considered for genetic engineering toward PHB production. For reasons surrounding the ease of genetic modification and characterization, Monsanto has selected corn as their crop of focus in the area of PHB production. This breed of Monsanto corn is harvested by conventional means, and used as a regular variety of the crop. The stover is also collected, and extraction of the PHB from the leaves and stalk is completed using a two-step alcohol extraction process.
Genetic engineering of mustard plants has been a research focus for Chris Somerville and his team at Stanford University. As early as 1990, they began working on a process by which the genes required to produce PHB would be accepted in the chloroplasts of mustard plant cells. The chloroplasts were selected as the host organelle for the PHB encoding genes, as this would prevent them from scavenging materials from other organelles which were required for the growth of the plant. Somerville and his co-researchers were able to produce plants which had a maximum of 20% (dry weight) PHB. As of the year 2000, researchers were still struggling with finding a balance between increasing PHB production by plants, and maximizing the plant's growth and fertility.
More recently, alfalfa has been chosen for genetic engineering toward PHB production due to its perennial growth, nitrogen-fixing capabilities, and the potential for multiple harvests in a single growing season. The organelles selected for PHB gene encoding in the alfalfa cells were the plastids. Although the agglomerations of PHB granules found in the alfalfa cells comprised a mere 0.2% of the plants dry weight, success was found in the passing of the PHB encoding genes between generations of the plants, which previous researchers had been unable to achieve.
Progress in the genetic engineering of plants has seen great successes in recent years, but public opinion of this practice is mixed. While complete utilization of plants (for both consumption and industrial applications) is seen as an ideal goal by many scientists, the general public tends to have reservations about consuming food which has been clearly genetically modified. In addition, although PHB is a completely biodegradable material, it lacks the structural integrity required for its sole use in polymer materials. It has a high decomposition level prior to melting, and results in a brittle material. However, the prospects for polymer production in conventional crops do both well for the initiatives of value-added processing, and whole crop utilization. In addition, the reduction in fossil fuels required to produce comparable plastic may be a satisfactory way to balance fears surrounding genetic modification of plants. This is an emerging technology, which will become more widely accepted as public education about it is expanded.
MICROBIALLY PRODUCED POLYMERS
Approximately 80 years ago, the first PHB polymer was isolated from a Bacillus magaterium bacteria cell. Since that time, biopolymer scientists have been attempting to find ways to expand and commercialize bacterial production of biopolymer materials. The common biopolymers which are able to be isolated from bacterial cells are polyhydroxyalkanoates (PHAs), polylactic acid (PLA), and PHBs, which have properties similar to those found in polyethylene and polypropylene. These biopolymers are found in nature, as intracellular deposits in bacteria, produced when bacteria must survive under unfavorable conditions. They act as an energy storage facility, and are developed when the bacteria's surroundings include excess carbon, and a deficiency of another nutrient. Bacterial production of PHAs and PHBs is based on fermentation processes using various materials as feedstock materials.
In a study by Wong et al. at Hong Kong Polytechnic University, PHB was produced by a bacteria (Staphylococcus epidermis) isolated from sesame oil processing waste. A number of materials were found to be suitable fermentation feedstocks for the bacteria, including pure fructose, barley malt, and even sesame oil itself. Pure fructose produced the highest PHB content in the bacterial cells, to a maximum of approximately 15% by dry weight. The PHB was stored as inclusion bodies in the bacterial cell cytoplasm, and was extracted by centrifugation and a multi-step washing process.
Throughout the 1990's, researchers at McGill University worked on developing a process by which the waste from potato chip processing could be used to microbially produce PHB. The potato waste was seen as a viable option as a feedstock for a niche market biopolymer, as it was available in large quantities at a very low cost. The potato processing waste contained a large amount of raw potato starch, which was treated by enzymatic hydrolysis to become a fermentable feedstock, appropriate as a substrate to form PHB using the bacteria Alcaligenes eutrophus. The potato starch was coupled with barley malt in a 90:10 blend to produce glucose, which supplied the necessary carbon source for the bacterial production of PHB molecules. A maximum of 77% (dry weight) PHB was recovered from the bacterial culture.
Polylactic acid (PLA) production is unique in its requirement of bacterial fermentation of a feedstock material (to produce lactic acid), followed by condensation of the lactic acid product. As outlined by Akerberg and Zacchi, the feedstock material is often a polysaccharide such as starch or cellulose. In a study completed at Kansas State University, a procedure for the production of lactic acid from grain sorghum has been developed. The procedure included cooking the grain sorghum, to facilitate gelatinization of its starch. The cooked sorghum was then used as a fermentation substrate for Rhizopus oryzae, which produced lactic acid at a concentration of approximately 25%. Zhan et al. also reported that a similar process using ground corn as the substrate has been successfully developed.
Until the late 1990's, researchers working in the development of microbial systems to produce biopolymers generally focused on using a single type of bacteria, with a minimum component feedstock. Recent work, however, has shifted to include systems where mixed microbial cultures are used with a multi-component feedstock system, to maximize cost effectiveness of the process. The greatest expense in producing biopolymers by these methods has traditionally been the substrate which is used as the fermentation feedstock. Being able to use a mixture of substrates, particularly if an exact formulation is not required, will greatly reduce these costs.
One example of a multi-component feedstock system, reported by Chua and Yu, employs activated sludge from a wastewater treatment plant as the fermentation feedstock. As an additional benefit of reducing feedstock cost, the given system also has positive environmental implications, as the bacterial action reduces the amount of further processing required on the activated sludge.
Japanese researchers have also developed a multi-component feedstock system for polymer production, but the polymer they have focused on is poly-l-lactate (PLLA); a bioplastic that gradually decomposes in average soil conditions. Their innovative process uses municipal food waste as a feedstock for the production of PLLA, thus reducing costs, and providing an environmentally conscious means of waste disposal. They consider their process to be the most practical one available, as there is no need to characterize the municipal waste prior to its use. The use of two types of bacteria (Propionibacterium freundenreichii and Lactobacillus rhamnosus) increases the efficiency of this process. The researchers have indicated that this process uses approximately 50% of the total available carbon content, and has a PLLA yield of approximately 7%.
Microbially-produced biopolymers are generally quite similar to those produced within genetically engineered plants. However, there are great differences between the two methods of their formation. In the case of microbial production of biopolymers, the factor inhibiting wider use of the practice is the high cost associated with feedstock materials, and the time required to produce the end product. The case has always been that it was less expensive and more economically feasible for industry to use fossil fuels to produce the mechanically comparable materials of PE and PP. However, now that multi-component feedstocks are gaining credibility, it is just a matter of time before the microbial production of polymer materials becomes commonplace.
Synthetic Biodegradable Polymers as Medical Devices
In the first half of this century, research into materials synthesized from glycolic acid and other ?-hydroxy acids was abandoned for further development because the resulting polymers were too unstable for long-term industrial uses. However, this very instability- leading to biodegradation-has proven to be immensely important in medical applications over the last three decades. Polymers prepared from glycolic acid and lactic acid have found a multitude of uses in the medical industry, beginning with the biodegradable sutures first approved in the 1960s. Since that time, diverse products based on lactic and glycolic acid-and on other materials, including poly(dioxanone), poly(trimethylene carbonate) copolymers, and poly (?-caprolactone) homopolymers and copolymers-have been accepted for use as medical devices. In addition to these approved devices, a great deal of research continues on polyanhydrides, polyorthoesters, polyphosphazenes, and other biodegradable polymers.
Why would a medical practitioner want a material to degrade? There may be a variety of reasons, but the most basic begins with the physician's simple desire to have a device that can be used as an implant and will not require a second surgical intervention for removal. Besides eliminating the need for a second surgery, the biodegradation may offer other advantages. For example, a fractured bone that has been fixated with a rigid, nonbiodegradable stainless implant has a tendency for refracture upon removal of the implant. Because the stress is borne by the rigid stainless steel, the bone has not been able to carry sufficient load during the healing process. However, an implant prepared from biodegradable polymer can be engineered to degrade at a rate that will slowly transfer load to the healing bone. Another exciting use for which biodegradable polymers offer tremendous potential is as the basis for drug delivery, either as a drug delivery system alone or in conjunction to functioning as a medical device.
Polymer scientists, working closely with those in the device and medical fields, have made tremendous advances over the last 30 years. This article will focus on a number of these developments. We will also review the chemistry of the polymers, including synthesis and degradation, describe how properties can be controlled by proper synthetic controls such as copolymer composition, highlight special requirements for processing and handling, and discuss some of the commercial devices based on these materials.
POLYMER CHEMISTRY
Biodegradable polymers can be either natural or synthetic. In general, synthetic polymers offer greater advantages than natural materials in that they can be tailored to give a wider range of properties and more predictable lot-to-lot uniformity than can materialize from natural sources. Synthetic polymers also represent a more reliable source of raw materials, one free from concerns of immunogenicity.
Table 1
Properties of Common Biodegradable Polymers
| Polymer | Melting Point (°C) | Glass- Transition Temp (°C) | Modulus (GPa)a | Degradation Time (months)b |
| PGA |
225-230 |
35-40 |
7.0 |
6 to 12 |
| LPLA |
173-178 |
60-65 |
2.7 |
] 24 |
| DLPLA |
Amorphous |
55-60 |
1.9 |
12 to 16 |
| PCL |
58-63 |
(-65)- (-60) |
0.4 |
]24 |
| PDO |
N/A |
(-10)-0 |
1.5 |
6 to 12 |
| PGA TMC |
N/A |
N/A |
2.4 |
6 to 12 |
| 85/15 DLPLG |
Amorphous |
50-55 |
2.0 |
5 to 6 |
| 75/25 DLPLG |
Amorphous |
50-55 |
2.0 |
4 to 5 |
| 65/35 DLPLG |
Amorphous |
50-55 |
2.0 |
3 to 4 |
| 50/50 DLPLG |
Amorphous |
45-50 |
2.0 |
1 to 2 |
a = Tensile or flexural modulus.
b = Time to complete mass loss. Rate also depends on part geometry.
The general criteria for selecting a polymer for use as a biomaterial is to match the mechanical properties and the time of degradation to the needs of the application. The ideal polymer for a particular application would be configured so that it:
- Has mechanical properties that match the application, remaining sufficiently strong until the surrounding tissue has healed.
- Does not invoke an inflammatory or toxic response.
- Is metabolized in the body after fulfilling its purpose, leaving no trace.
- Is easily processable into the final product form.
- Demonstrates acceptable shelf life.
- Is easily sterilized.
The factors affecting the mechanical performance of biodegradable polymers are those that are well known to the polymer scientist, and include monomer selection, initiator selection, process conditions, and the presence of additives. These factors in turn influence the polymer's hydrophilicity, crystallinity, melt and glass-transition temperatures, molecular weight, molecular-weight distribution, end groups, sequence distribution (random versus blocky), and presence of residual monomer or additives. In addition, the polymer scientist working with biodegradable materials must evaluate each of these variables for its effect on biodegradation.
Biodegradation has been accomplished by synthesizing polymers that have hydrolytically unstable linkages in the backbone. The most common chemical functional groups with this characteristic are esters, anhydrides, orthoesters, and amides. We will discuss the importance of the properties affecting biodegradation later in the article.
The following section presents an overview of the synthetic biodegradable polymers that are currently being used or investigated for use in wound closure (sutures, staples); orthopedic fixation devices (pins, rods, screws, tacks, ligaments); dental applications (guided tissue regeneration); cardiovascular applications (stents, grafts); and intestinal applications (anastomosis rings). Most of the commercially available biodegradable devices are polyesters composed of homopolymers or copolymers of glycolide and lactide. There are also devices made from copolymers of trimethylene carbonate and ?-caprolactone, and a suture product made from polydioxanone.
Polyglycolide (PGA). Polyglycolide is the simplest linear aliphatic polyester. PGA was used to develop the first totally synthetic absorbable suture, marketed as Dexon in the 1960s by Davis and Geck, Inc. (Danbury, CT). Glycolide monomer is synthesized from the dimerization of glycolic acid. Ring-opening polymerization yields high-molecular-weight materials, with approximately 1-3% residual monomer present. PGA is highly crystalline (45-55%), with a high melting point (220-225°C) and a glass-transition temperature of 35-40°C. Because of its high degree of crystallization, it is not soluble in most organic solvents; the exceptions are highly fluorinated organics such as hexafluoroisopropanol. Fibers from PGA exhibit high strength and modulus and are too stiff to be used as sutures except in the form of braided material. Sutures of PGA lose about 50% of their strength after 2 weeks and 100% at 4 weeks, and are completely absorbed in 4-6 months. Glycolide has been copolymerized with other monomers to reduce the stiffness of the resulting fibers.
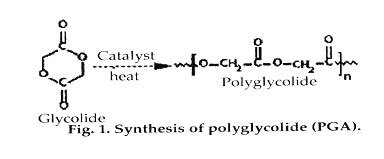
Polylactide (PLA). Lactide is the cyclic dimer of lactic acid that exists as two optical isomers, d and I. l-lactide is the naturally occurring isomer, and dl-lactide is the synthetic blend of d-lactide and l-lactide. The homopolymer of l-lactide (LPLA) is a semicrystalline polymer. These types of materials exhibit high tensile strength and low elongation, and consequently have a high modulus that makes them more suitable for load-bearing applications such as in orthopedic fixation and sutures. Poly(dl-lactide) (DLPLA) is an amorphous polymer exhibiting a random distribution of both isomeric forms of lactic acid, and accordingly is unable to arrange into an organized crystalline structure. This material has lower tensile strength, higher elongation, and a much more rapid degradation time, making it more attractive as a drug delivery system. Poly(l-lactide) is about 37% crystalline, with a melting point of 175-178°C and a glass-transition temperature of 60- 65°C. The degradation time of LPLA is much slower than that of DLPLA, requiring more than 2 years to be completely absorbed. Copolymers of l-lactide and dl-lactide have been prepared to disrupt the crystallinity of l-lactide and accelerate the degradation process.
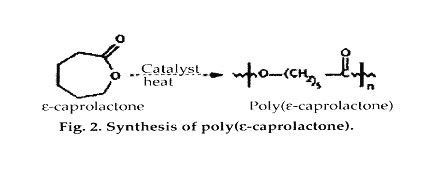
Poly(?-caprolactone). The ring-opening polymerization of ?-caprolactone yields a semicrystalline polymer with a melting point of 59-64°C and a glass-transition temperature of-60°C. The polymer has been regarded as tissue compatible and used as a biodegradable suture in Europe. Because the homopolymer has a degradation time on the order of 2 years, copolymers have been synthesized to accelerate the rate of bioabsorption. For example, copolymers of ?-caprolactone with dl-lactide have yielded materials with more-rapid degradation rates. A block copolymer of ?-caprolactone with glycolide, offering reduced stiffness compared with pure PGA, is being sold as a monofilament suture by Ethicon, Inc. (Somerville, NJ), under the trade name Monacryl.
Poly(dioxanone) (a polyether-ester). The ring-opening polymerization of p-dioxanone resulted in the first clinically tested monofilament synthetic suture, known as PDS (marketed by Ethicon). This material has approximately 55% crystallinity, with a glass-transition temperature of -10 to 0°C. The polymer should be processed at the lowest possible temperature to prevent depolymerization back to monomer. Poly(dioxanone) has demonstrated no acute or toxic effects on implantation. The monofilament loses 50% of its initial breaking strength after 3 weeks and is absorbed within 6 months, providing an advantage over Dexon or other products for slow-healing wounds.
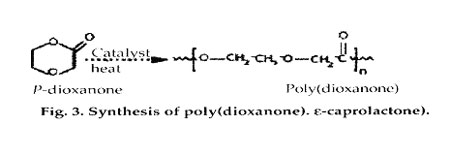
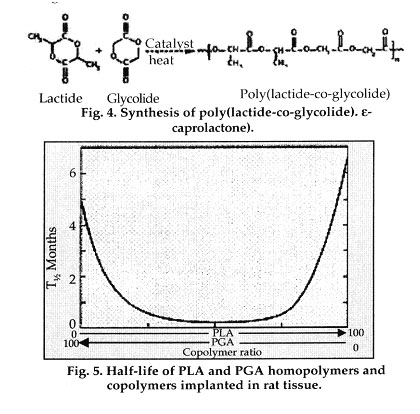
Poly(lactide-co-glycolide). Using the polyglycolide and poly(l-lactide) properties as a starting point, it is possible to copolymerize the two monomers to extend the range of homopolymer properties. Copolymers of glycolide with both l-lactide and dl-lactide have been developed for both device and drug delivery applications. It is important to note that there is not a linear relationship between the copolymer composition and the mechanical and degradation properties of the materials. For example, a copolymer of 50% glycolide and 50% dl-lactide degrades faster than either homopolymer Copolymers of l-lactide with 25-70% glycolide are amorphous due to the disruption of the regularity of the polymer chain by the other monomer. A copolymer of 90% glycolide and 10% l-lactide was developed by Ethicon as an absorbable suture material under the trade name Vicryl. It absorbs within 3-4 months but has a slightly longer strength-retention time.
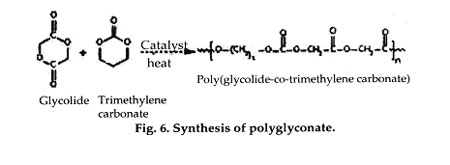
Copolymers of glycolide with trimethylene carbonate (TMC), called polyglyconate have been prepared as both sutures (Maxon, by Davis and Geck) and as tacks and screws (Acufex Microsurgical, Inc., Mansfield, MA). Typically, these are prepared as A-B-A block copolymers in a 2:1 glycolide:TMC ratio, with a glycolide-TMC center block (B) and pure glycolide end blocks (A). These materials have better flexibility than pure PGA and are absorbed in approximately 7 months. Glycolide has also been polymerized with TMC and p-dioxanone (Biosyn, by United States Surgical Corp., Norwalk, CT) to form a terpolymer suture that absorbs within 3-4 months and offers reduced stiffness compared with pure PGA fibers.
Other Polymers under Development. Currently, only devices made from homopolymers or copolymers of glycolide, lactide, caprolactone, p-dioxanone, and trimethylene carbonate have been cleared for marketing by FDA. A number of other polymers, however, are being investigated for use as materials for biodegradable devices.
In addition to their suitability for medical uses, biodegradable polymers make excellent candidates for packaging and other consumer applications. A number of companies are evaluating ways to make low-cost biodegradable polymers. One method is to bioengineer the synthesis of the polymers, using microorganisms to produce energy-storing polyesters. Two examples of these materials-polyhydroxybutyrate (PHB) and polyhydroxyvalerate (PHV)-are commercially available as copolymers under the trade name Biopol and have been studied for use in medical devices. The PHB homopolymer is crystalline and brittle, whereas the copolymers of PHB with PHV are less crystalline, more flexible, and easier to process. These polymers typically require the presence of enzymes for biodegradation but can degrade in a range of environments and are under consideration for several biomedical applications.
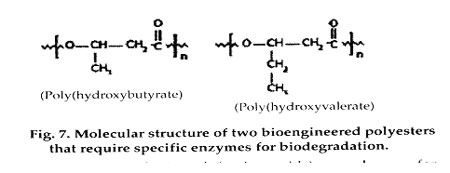
The use of synthetic poly(amino acids) as polymers for biomedical devices would seem a logical choice, given their wide occurrence in nature. In practice, however, pure insoluble poly(amino acids) have found little utility because of their high crystallinity, which makes them difficult to process and results in relatively slow degradation. The antigenicity of polymers with more than three amino acids in the chain also makes them inappropriate for use in vivo. To circumvent these problems, modified "pseudo" poly(amino acids) have been synthesized by using a tyrosine derivative. Tyrosine-derived polycarbonates, for example, are high-strength materials that may be useful as orthopedic implants. It is also possible to copolymerize poly(amino acids) to modify their properties. The group that has been researched most extensively is the polyesteramides.
A Note on Nomenclature
A polymer is generally named based on the monomer it is synthesized from. For example, ethylene is used to produce poly(ethylene). For both glycolic acid and lactic acid, an intermediate cyclic dimer is prepared and purified, prior to polymerization. These dimers are called glycolide and lactide, respectively. Although most references in the literature refer to polyglycolide or poly(lactide), you will also find references to poly(glycolic acid) and poly(lactic acid). Poly(lactide) exists in two stereo forms, signified by d or I for dexorotary or levorotary, or by dl for the racemic mix.
The search for new candidate polymers for drug delivery may offer potential for medical device applications as well. In drug delivery, the formulation scientist is concerned not only with shelf-life stability of the drug but also with stability after implantation, when the drug may reside in the implant for 1-6 months or more. For drugs that are hydrolytically unstable, a polymer that absorbs water may be contraindicated, and researchers have begun evaluating more hydrophobic polymers that degrade by surface erosion rather than by bulk hydrolytic degradation. Two classes of these polymers are the polyanhydrides and the polyorthoesters.
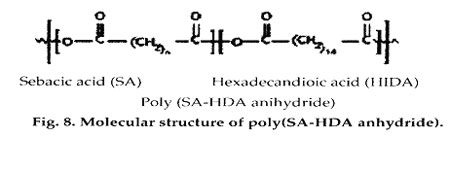
Polyanhydrides have been synthesized via the dehydration of diacid molecules by melt polycondensation. Degradation times can be adjusted from days to years according to the degree of hydrophobicity of the monomer selected. The materials degrade primarily by surface erosion and possess excellent in vivo compatibility. So far, they have only been approved for sale as a drug delivery system. The Gliadel product, designed for delivery of the chemotherapeutic agent BCNU in the brain, received regulatory clearance from FDA in 1996 and is being produced by Guilford Pharmaceuticals, Inc. (Baltimore).
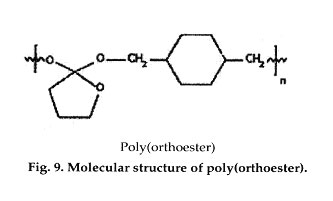
Polyorthoesters were first investigated in the 1970s by Alza Corp. (Palo Alto, CA) and SRI International (Menlo Park, CA) in a search for new synthetic biodegradable polymers for drug delivery application. These materials have gone through several generations of improvements in synthesis, and can now be polymerized at room temperature without forming condensation by-products. Polyorthoesters are hydrophobic, with hydrolytic linkages that are acid-sensitive but stable to base. They degrade by surface erosion, and degradation rates can be controlled by incorporation of acidic or basic excipients.
Biodegradable Plastics from Renewable Sources
ANALYSIS
Plastics and the environment
It is increasingly being realized that the use of long-lasting polymers for short-lived applications is not entirely justified, especially when increased concern exists about the preservation of living systems. The elimination of waste plastics is therefore of interest in surgery, hygiene, catering, packaging, agriculture, fishing, environmental protection, technical and other potential applications.
Most of today's plastics and synthetic polymers are produced from petrochemicals. As conventional plastics are persistent in the environment, improperly disposed plastic materials are a significant source of environmental pollution, potentially harming wildlife. In seas, for example, plastic rubbish -from ropes and nets to the plastic bands from beer packs- chokes and entangles marine mammals. One study on cetacean carcasses found that 1 in 30 had choked on plastic debris. Plastics have also a costly impact on waste management, and municipalities are becoming aware of the significant savings that collection of 'wet' organic wastes in so-called 'biobins' to be composted can provide. For these reasons, reaching the conditions for replacement of non-degradable polymers by degradable plastics, particularly for single-use disposables and packaging applications, is of major interest both to decision-makers and the plastic industry.
The move to renewable sources
The production of biodegradable plastics can be viewed within the wider context of the 'greening of industry', with most national RTD programmes viewing the use of renewable biomass as an alternative feedstock to fossil fuels in chemical manufacturing. The chief reasons for this are the exploiting the potential of photosynthesis for energy savings, curbing the greenhouse effect, developing eco-compatible processes & products, diversifying agriculture out of food production and, possibly, generating employment.
Thus both renewable biomass feedstocks (crops) and agro-industrial 'waste' streams emerge as the key alternatives. Indeed, wastes offer the greatest promise as a feedstock: not only are they cheap, but their conversion solves another environmental problem by turning 'waste' into useful products. This means that countries without scope for crop expansion could still benefit from the approach, both economically and ecologically, by reducing the environmental impact associated with the disposal of their industrial wastes. This has led to substantial research, and also very recently resulted in some innovations in two main directions, namely the development of new microbes that can convert cheap substrates, and the cloning and expression of biosynthetic genes in plants.
Extending the recycling loop
Recycling of conventional plastics is a way of reducing the problems associated with plastic waste. However, many packaging materials do not lend themselves to recycling owing to contamination with food and ink, and the necessary cleaning prior to recycling is very expensive. Packaging recycling costs Germans about DM3 per kg. Furthermore, reprocessing often leads to a downgrading of the polymer use and an increased hold-up in the system. A lack of markets for recycled polymers has led to large stockpiles and the dumping of waste products in other countries. A recent change in German legislation concerning packaging recycling, dropping quotas from the initial 64% to 50%, is largely a response to the lack of market for low quality recycled polymers. Depolymerization technologies are being developed that can return the plastics to its starting material (feed monomer) so that it can then be used to remake the non-degradable polymer. However this approach will increase the cost of the feed monomer and hence the final plastic, and does not solve the degradability problem for the plastic that still ends up into the environment.
Even the more efficient recycling loop of non-biodegradable plastic waste in any production process has an associated production cost, to which the disposal cost must be added. Biodegradable plastics are, however, not to be seen as replacement for plastics, but only for specific applications. In fact, their unique physical properties mean that some plastic materials will remain unsubstituted for a long time.
Biopolymers, conventional plastics and biodegradable plastics
The resins used to make biodegradable plastics fall into two broad categories: natural and synthetic. Natural resins (or biopolymers) are largely based on renewable resources such as starch and cellulose, and polyhydroxyalcanoates (PHA) produced by microbes. Other polymers such as proteins and pectins may also potentially be developed for biodegradable plastics and polymers. Polylactides (PLA), i.e. aliphatic polyesters formed by polymerization of lactic acid is usually included in this category since the monomer can be produced by fermentation.
Synthetic polymers are made of petroleum-based and other feedstocks and include polyester and polyethylene polymers. An example of a biodegradable, synthetic polymer is polycaprolactone, a thermoplastic polyester resin. Conversely, physical or chemical modification of a natural biopolymer may result in a loss of its biodegradability. Conventional, petrochemical-based plastic materials are not easily degraded in the environment because of their high molecular weight an hydrophobic character. Disposal of plastics, therefore has become a major environmental concern, resulting in programmes to recycle, incinerate or compost these wastes.
The plastics sector
Consumption of plastics material in the EU plastics processing industry was approximately 30 million tonnes in 1994. Germany accounts for nearly one-quarter of total EU demand. Asian economies are becoming more integrated and less dependent on the US and Europe. Japan, Korea, Taiwan, China and Singapore are the main plastic processing countries in Asia with production of more than 25 million tonnes; i.e. one-quarter of world production. China's output of plastic products has been growing at an annual rate of over 20% during the 1990s. In recent years, EU exports to central and eastern Europe countries have increased tremendously. Consequently, waste management problems are expected to worsen in these countries, some of which may possibly join the EU in the near future. In highly-industrialized countries plastics represent between 20 and 40% of municipal solid waste by volume. The time has therefore come to think of cooperation in developing strategies for degradables, similarly to what is happening in Asian countries, and especially because eastern and central European countries possess the abundant agricultural resources which are needed to produce the feedstock.
Packaging
Accounting for one-third of demand, the production of packaging material is the largest subsector of the plastics processing industry. The food industry constitutes the major end-user followed by the distribution and beverage industries. Despite environmental concerns, the European market for plastic packaging is rising by billions of ECUs every year. Pharmaceuticals, toiletries and cosmetics are large users of packaging. Hence, to keep abreast of future restrictive legislation aimed at reducing packaging weight and volume, these industries are very interested in seeing cheap biodegradable packaging available on the market.
The greatest growth rate has been predicted for polyester bottle resins, particularly in the carbonated drinks market, whose annual demand now stands at more than 500 million ECU. In response to this, Japanese companies have recently designed a substitute for polyester bottles with excellent physical properties by modifying 'Bionolle', a biodegradable polyester, using a technique known as stretch-blow moulding.
Plastic films
Plastic films are mostly based on polyethylenes with LDPE accounting for one-sixth of total EU plastics consumption. Major end-uses of plastic films are printed films for automatic packaging, shrink and stretch films for overpacking, films for agriculture and horticulture (greenhouses, mulching), films for construction, shoppers, carrier bags, refuse bags, heavy duty sacks, and films for a wide range of technical applications such as magnetic tapes, credit cards, hot foil stamping, cables, motor insulation, furniture films and office films. Agriculture accounts for 3% of total plastic consumption in Europe. Plastic films for covering greenhouses have enjoyed both innovation and spectacular growth over the last twenty years.
Structure of the business
The plastics industry exists within the broader context of the petrochemical industry. This sector has recently been hard hit by the need to direct significant amounts of capital expenditure and R&D funding in order to comply with ever stricter environmental regulations. Existing capacity for polymer production is currently in the hands of the main petrochemical groups, which in turn respond to the demand for raw materials by processors. The plastics processing industry (converters) is mainly composed of small and medium-sized companies. After procurement of the raw material, the medium-sized plastics processor has to sell plastic products through large scale industries, like car makers, manufacturers of electric and electronic equipment and department store chains.
Biodegradable polymer technology can at present only offer a limited range of materials. This is the main reason why US and Japan are now focusing on the technological development of biodegradable polymers, in order to expand the range of these polymers that can fulfil processing and property requirements for many applications in which biodegradability would be an important materials property. The biochemical industry (food, grain, sugar) is therefore in an ideal position to build capacity for biodegradable plastics at the expense of the petrochemical industry or, conversely, the petrochemical industry could adapt its technology for processing renewable feedstocks, thereby profiting from a long-standing process experience. In the transition, there exists a niche in which SMEs that integrate both polymers production and processing could emerge.
Recent developments
Space does not permit a review of all biodegradable plastics types existing or under-development. In what follows only two recent examples of innovation are mentioned.
Four US DOE (Department of Energy) labs have signed a $7 million agreement with Applied CarboChemicals, a specialty chemicals company, to manufacture chemical feedstocks from renewable farm crops at a significant lower cost than via conventional petroleum-based chemosynthesis. The project follows the recent DOE development of a new microbe as part of a process that converts corn into the key intermediaries used to make a range of industrial and consumer products, including polymers, clothing fibres, paints, inks, food additives, automobile bumpers. Existing domestic markets for such chemicals total more than $1.3 billion a year. A rise in the number of employees is also expected over the next decade as the company builds manufacturing capacity and expands into global markets. This is a typical example of combining chemosynthesis with biosynthesis plants, an approach pursued in America to save energy. The R&D line being followed suggests the possibility that in the near future the process might be extended to use industrial organic wastes (such as from the sugar industry) to replace corn.
Metabolix (Cambridge, Mass) has recently licensed MIT's patents on the insertion of the genes for the production of the key enzymes in the mechanism of production of PHB (polyhydroxybutyrate - an essential component of biodegradable polyester thermoplastics) into bacteria and transgenic corn. The transgenic bacteria and plants can also co-polyesterify ß-hydroxybutyrate with ß-hydroxyalcanoates up to C12. As so far PHB  the only PHA shown to be produced in plants  has poor physical properties, and attempts to blend it with other polymers and plasticizers have had only limited success, these innovations would seem to be essential for a broader use of PHA in commodity products. The exciting potential of the production of biodegradable plastics in a low cost, renewable production system (using corn, cassava, soybean, etc.) is also apparent from the spate of recent joint-ventures as well as business purchases by big multinational commodity firms, like Monsanto and Cargill. Table l refers to some of the pre-commercial and commercial work that is going on in the EU.
Previous false starts in the development of biodegradable materials have foundered on account of high costs, absence of good physical properties (mainly water barrier and heat resistance), lack of an adequate infrastructure for waste management and an adequate means whereby the public can differentiate products.
Table 1
Biodegradable Plastics in Europe: Business examples
| Company | Activities |
| Biotec (Melitta) Emmerich (Germany) |
Commercial business/production of biodegradable starch/polycaprolactone (PCL) compounds for e.g. packaging and waste handling (refuse bags) applications. |
| BASF Ludwigshafen (Germany) |
Development of synthetic, biodegrable co-polyesters and blends with starch e.g. for flexible films applications. |
| Bayer/Wolf Walsrode Leverkusen (Germany) |
Pre-commercial synthetic, biodegradable co-polyester amide for e.g. flexible film applications. |
| Novamont Novara (Italy) |
Commercial business/ production of starch compounded with poly-caprolactone and/or polyvinylalcohol;' Mater-Bi'. |
Biodegradability and compostability
Certain blends of polyethylene and starch can be degraded by physical agents (such as light). Indeed, a type of polyethylene is being marketed that includes a catalyst prompting the polymer's thermal degradation. Nevertheless, biodegradation is quite another thing .
ASTM standard D-5488-94d defines biodegradable as "capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, or biomass in which the predominant mechanisms is the enzymatic action of micro-organisms, that can be measured by standard tests, in a specified period of time, reflecting available disposal conditions".
Composting is an accelerated biological decay process viewed by many to be a potential solution to the solid-waste management crisis existing in many parts of the world. Compostable is defined as "capable of undergoing biological decomposition in a compost site as part of an available program, such that the material is not visually distinguishable and breaks down to carbon dioxide, water, inorganics and biomass, at a rate consistent with known compostable materials."
Management of solid waste should include a critical understanding of the fate of synthetic polymers which may be disposed as solid waste in municipal landfills. Research, marketing and regulatory reviews of degradable polymers should take into account the characteristics of true landfills-not just lab tests of degradation.
To meet the compostability requirement, all of the blend components have to fully biodegrade under composting conditions and within the timeframe of the composting process. Draft national and European test standards for measuring biodegradability under composting conditions are currently under development. The key issue is whether the biodegradation material (ie the residue left by biodegradation) is harmful to the environment. Testing the amount of mineralization alone does not take into account the nature of the residue left. Furthermore, biodegradation of blends of non-degradable synthetic polymers and starches, which can actually 'biodisintegrate', is doubtful.
Germany is dealing with the issue of plant health in its biodegradability/composting standards; 'a product must be fully biodegradable under composting conditions and the compost material cannot be phytotoxic or ecotoxic  it will support plant and microbial activity. In fact, the assumption that using natural ingredients always leads to harmless products is not true. Most important is the final destination of the biodegradable material.
One issue to be addressed is if current laboratory tests accurately reflect the biodegradability of a material in an real compost pile. The environments in which biodegradation takes place differ widely in terms of microbial composition, pH, temperature and moisture and they are not readily reproduced in the laboratory. Another issue for standards development is balancing the need for shelf life with the demand for rapid degradability. The development of more sophisticated distribution systems so as to avoid products sitting in warehouses, and the creation of more composting facilities directly related to the disposal or these products would be needed. In Japan, the Biodegradable Plastics society (BPS) has proposed a standard for degradability that has been accepted there and is being considered by the International Standards Organization.
The OK Compost Conformity Mark is awarded jointly by the international quality inspection bureau A.I.B.-Vinçotte Inter and Organic Waste System, a research institute in the field of biodegradability. Manufacturers can use this label on their material as a proof that it passes the biodegradability test and is appropriate to compost. So far, no internationally adopted standard laboratory method exists for investigating aerobic biodegradability in a composting environment.
Challenges ahead
Acceptance of biodegradable polymers is likely to depend on four unknowns: (1) customer response to costs that today are generally 2 to 4 times higher than for conventional polymers; (2) possible legislation (particularly concerning water-soluble polymers); (3) the achievement of total biodegradability; and (4) the development of an infrastructure to collect, accept, and process biodegradable polymers as a generally available option for waste disposal.
In a social context biodegradable plastics call for a re-examination of life-styles. They will require separate collection, involvement of the general public, greater community responsibility in installing recycling systems, etc. On the question of cost, awareness may often be lacking of the significance of both disposal and the environmental costs which are to be added to the processing cost.
Biodegradability is tied to a specific environment. For instance, the usual biodegradation time requirement for bioplastic to be composted is 1 to 6 months. In Europe, composting is on the increase, and the percentage of population with composting facilities available for their rubbish stands at about 80% in the Netherlands, 40% in Germany, and 30% in Belgium. Adequate regulation is still lacking however, and complaints have already appeared, for example in the Netherlands, where citizens must pay the same tax for plastics that go to composting as for those that go to incineration.
The development of starch-based biodegradable plastics looks very promising given the fact that starch is inexpensive, available annually, biodegradable in several environments and incinerable. The main drawbacks the industry is running into are bioplastics' low water-barrier and the migration of hydrophilic plasticizers with consequent ageing phenomena. The first problem together with the cost factor is common to all other biodegradable plastics.
As far as biological polyesters (PHA) are concerned, the recent purchase of Zeneca's Biopol business by Monsanto, who aims to expand it to include plant-derived polymers, does not suggest a bright future for microbial production of these polymers. Nevertheless, research on the production of the polymers by bacteria is worthwhile because it may be useful in helping us understand how to expand the range of polymers made by plants.
In summary, the bioplastics of the future will be produced from renewable sources, will have a low energy content and will display in-use properties similar to those of conventional plastics.
Process for the Preparation of Biodegradable Synthetic Polymers
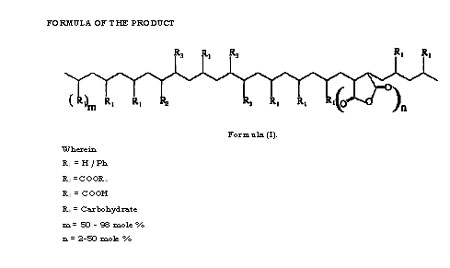
The present invention relates to the said products and the process for the preparation thereof through introduction of carbohydrate molecules. More particularly it relates to developing and synthesizing biodegradable polymers based on synthetic polymers,particularly polyolefins and their copolymers, and any other chemical modifications of polyolefms, and chemically linking carbohydrate molecules onto these base synthetic polymers.
INTRODUCTION
Most of the earlier work on synthesis of polymers with synthetic backbone and pendant carbohydrate units was based on either homopolymerization of vinylsaccharide monomers, or their copolymerization with other vinyl monomers. Synthesis of vinylsaccharide monomers had many disadvantages for commercial exploitation such as multi-step synthesis, including protection and deprotection of the carbohydrate monomers, isolation of intermediates, use of a large inventory of specialized chemicals, and so on. Copolymerization of vinyl monomers with vinylsaccharides is limited by the reactivity ratios, in addition to the usual other disadvantages mentioned for homolpolymers of vinyl carbohydrates. Therefore these methods are unsuitable and economically unviable, and inspite of several years of development, have not been commercialized for use as bulk plastics.
Due to the excellent processing properties, polyolefins have occupied a special status as commodity plastics. However the major drawback of polyolefins is that they are non- biodegradable and hence pose severe problems of their disposal after their useful life. With the aim of developing biodegradable polymers based on polyolefins, blending of starch with polyolefins (particularly with polyethylene) has been much explored and also put in practice in a limited way.
The intention of such blending procedures was that after disposal, degradation of starch in the blend would create voids and weaken the integrity of the polyethylene and result in its degradation. However the main drawback of this methodology is that attainment of such properties demands larger volumes of starch (in the range of 30% or higher) due to which the physical properties of the polyethylene have to be compromised.
Polystyrenes with pendant carbohydrate residues have been synthesized by polymerization of vinyl benzyl sugars. Polystyrene derivatives with maltose, lactose and maltotriose substituents on each phenyl ring were synthesized by coupling the corresponding oligosaccharide lactones with p-vinylbenzyl amines followed by radical polymerization. These polymers are water soluble, and are potential biomedical materials wherein the oligosaccharide moieties are used as recognition signals. However, there is no scope to develop these into bulk plastics as substitutes for polyolefins. Carbohydrates have also been incorporated into silicone rubbers in order to improve their wettability and biocompatibility. Allyl ethers of the protected carbohydrates were used as additives in the crosslinking of H-Si-polysiloxanes and vinyl-Si- polysiloxanes followed by deprotection of the protecting groups. The synthesis, products and the applications are quite different from the current patent proposal. Inert polyolefins such as polypropylene were hydrophilized with carbohydrate (carbohydrate azides) using UV radiations in acetone solvent. This procedure involved only the surface modification to make the surface hydrophilic so as to facilitate cell adhesion, to improve the dyeability, printability and biocompatability of the polymers. The synthesis, products and the applications are quite different from the current patent proposal.
Composites of polypropylene (PP) and cellulose were synthesized by kneading the two components in presence of a compatibilizer viz. polypropylene-maleic anhydride in an extruder in an attempt to obtain potential biodegradable polymers. The synthesis and structure of these polymers are quite different from the current patent proposal. A biodegradable polyolefin based on carbohydrates has been synthesized wherein the synthetic polymeric backbone is poly(vinyl alcohol) and the carbohydrate component is glucose. The glucose is linked to the polymer via a spacer viz. adipic acid. The monomer, viz. the vinyl carbohydrate, was synthesized enzymatically whereas the polymerization was carried out by chemical means. The polymers with lower molecular weights (Mn 3600 and 7000) were degraded to 70% in 28 days whereas polymers with high molecular weights (Mn 12900 and 34400) degraded less. Polyvinyl alcohol degrading bacteria such as Pseudomonas, Bacillus megaterium and Alcaligenes faecalis were used for the biodegradation tests. It is well known that poly(vinly alcohol) itself is a biodegradable polymer, and the synthesis, products and the applications of this system are quite different from the current patent proposal.
Glucosamine hydrochloride and galactosamine hydrochloride were grafted onto polyacryloyl chloride by polymer analogous reactions in carbonate buffer (Bahulekar R., Tokiwa t, Kano J., Matsumura T., Kojima I., Kodama M., Carbohydrate Polymers, 37, 71-78). The synthesis, products and the applications are quite different from the current patent proposal. The aim was not to develop processable polyolefins for any usual applications of polyolefins, and the methods were not tailored for developing such methods. Other reports of linking carbohydrates onto synthetic polymer backbones have also been found in literature. Sucrose was attached to low molecular weight carboxylated polybutadiene and utilized for synthesis of polyurethanes. In another study, surface modification of PVC films was carried out with sucrose to make the surface of the polymer wettable and as a carbon source for microorganisms. This is quite different from making the entire polymer a carbon source by modifying with carbohydrates (as in the case of our report) rather than just modifying the surface of the polymer.
Polyethylene containing prooxidant and 6% starch was reported to be biodegradable. Pure cultures of Streptomyces baduis, S virido sporus and S. Setonii were used for the biodegradation tests. The films were either chemically disinfected or irradiated by UV or were thermally treated prior to the biodegradation tests. However, these are blends of polymers and not discrete carbohydrate linked polymer chains.
Carbohydrate based copolymers are also reported. Copoly(ester amide)s were prepared by random copolymerization of protected carbohydrates. A very recent comprehensive review of biodegradable polymers in one of the world's best known journals also does not mention research results of the type mentioned in this patent proposal.
OBJECTIVE OF THE PRESENT INVENTION
The present invention, which relates to developing and synthesizing biodegradable polymers based on synthetic polymers, particularly polyolefins and their copolymers, and any other chemical modifications of polyolefins, by chemically linking carbohydrate molecules onto these synthetic polymers has many advantages over the earlier methods described above. In the present invention no protection of the carbohydrates is required. The reaction conditions are mild. Even few number of carbohydrate molecules being attached to the synthetic polymers (less than 5% by weight) brings about greatly enhanced rates of biodegradation. It is not at all obvious that presence of a few molecules (as low as 5% by weight) can cause a significant increase in the rates of biodegradation of these polymers. The further advantage of having very low number of carbohydrate molecules attached to the polymer chain is that the physical/mechanical properties of the base synthetic polymer are not greatly affected. Further, the advantage of less number of synthetic steps and mild reaction conditions is ease of technology development and subsequent implementation.
The main objective of the present invention therefore is to provide biodegradable polymers and the preparation thereof by covalently linking minute quantities of carbohydrate molecules onto base synthetic polymers, in particular polyolefins and their copolymers.
Another object is to use unprotected carbohydrate residues thereby avoiding tedious multi-step synthesis of the carbohydrate monomers.
Yet another object is to utilize very mild reaction conditions to obtain the above mentioned biodegradable polymers.
Still another object is to render non-biodegradable polyolefins biodegradable by anchoring minute quantities of carbohydrates onto the polymers.
Accordingly the present invention provides biodegradable synthetic polymers having a formula
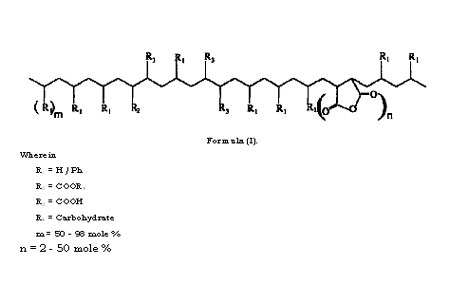
The present invention also provides a process for the preparation of biodegradable synthetic polymers of formula (I), which comprises drying a base synthetic polymer and a carbohydrate in vacuum at a temperature ranging between 55 to 60°C for a period of 17 to 19 hours, preparing separately the solutions of the base synthetic polymer in dry organic solvent, preparing a solution of a carbohydrate and a catalyst in dry organic solvent, adding the solution of the base synthetic polymer into the solution of carbohydrate and the catalyst under agitation, heating the reaction mixture to a temperature ranging between 25 to 110°C for 2- 48 hours under nitrogen and agitation, cooling the reaction mixture to room temperature, precipitating the product using a solution of an inorganic salt in a non-solvent, washing the product with a solvent till the product is free from the salt to obtain the product.
PREFERRED EMBODIMENTS
In one of the embodiments of the present invention the base synthetic polymer may be a polyolefin and more preferably polystyrene, functionalized with reactive groups such as carboxyl, hydroxyl, amino, alkene, halide, ester, acid chloride and preferably anhydride.In another embodiment the carbohydrate may be monosaccharides, and disaccharides: both reducing and non-reducing and oligosaccharides, wherein none of the hydroxyls are protected, or carbohydrates in which at least one but not all hydroxyls have been protected.
In another embodiment the dry organic solvent used for the preparation of the polymer solution may be dry N,N'-dimethylformamide, dry pyridine or dry toluene and their mixtures.
In still another embodiment the catalyst may be pyridine, 4-dimethylaminopyridine, para-toluenesulfonic acid and carbodiimide catalysts.
In still another embodiment, the dry organic solvent used for the preparation of solution of carbohydrate and the catalyst may be dry N,N'- dimethylformamide or dry pyridine and their mixtures.
In yet another embodiment the molar ratio of base synthetic polymer to the carbohydrate in the reaction mixture may be 1: 0.01 or 1:1 or 1:2 or 1: 6, but preferably 1: 0.5 or more preferably 1:3.
In yet another embodiment the molar ratio of the functional groups on the base synthetic polymer and the catalyst may be 1: 0.01 upto 1: 2, but preferably 1: 0.14.
In still another embodiment the non-solvents used for the preparation of the salt solution may be water, ethanol, methanol or acetone and their mixtures.
In yet another embodiment the salts used for the preparation of the salt solution may be sodium or potassium salts, preferably sodium chloride, potassium chloride, sodium bromide, potassium bromide and most preferably sodium chloride.
In still another embodiment the solvents used for washing the product to make it free from salt may be water, ethanol, methanol or acetone and their mixtures.
In a feature of the present invention the process for rendering polystyrene biodegradable comprises of reacting functionalized polystyrene with monosaccharides, disaccharides and oligosaccharides without the protection of the hydroxyl groups, in dry organic solvents in presence of catalysts and in inert atmosphere.
In further feature in case of functionalized polystyrene e.g., polystyrene functionalized with maleic anhydride, dissolved in dry N,N'-dimethylformamide, was added to a stirred solution of the carbohydrate e.g., glucose, sucrose, lactose, methyl glucoside, etc. and the catalyst viz. 4-dimethylaminopyridine in a three-necked round bottom flask provided with a magnetic stir bar, thermowell, addition funnel and a dry nitrogen balloon at a temperature 45-65°C over a period of 1/2 -1 hour. The mole ratio of the maleic anhydride content of the polystyrene-maleic acid copolymer versus the carbohydrate was either 1: 3 or 2:1. Stirring was done for 4 - 18 hours at temperatures of 45 - 65°C. The amount of carbohydrate incorporated into the polymer by this method was generally between 0.5 to 5% by weight. The reaction mixtures were precipitated in brine, washed several times with water till free of chloride and dried in a vacuum oven.
These polymers were found to be degraded by both bacterial as well as fungal cultures.
Samples of polymers were taken with pure bacterial cultures such as Pseudomonas sp., Bacillus sp., etc. in flasks containing salts and no other source of carbon. The growth of the microorganisms was monitored by optical density measurements. The degradation was evidenced by weight losses and was also supported by spectral methods (IR spectoscopy). Similarly, the fungal cultures consisted of Aspergillius niger, P. ochro-chloron, Trichoderma sp., Pullularia pullulans, etc.
The present invention is illustrated using polystyrene-co-maleic anhydride (PSMAH) as the base synthetic polymer; lactose, D-glucose, sucrose and methyl glucoside as the carbohydrates; and 4- N,N'- dimethylaminopyridine (4-DMAP) as the catalyst. It is pertinent to note that the working of the present invention is not limited to the base synthetic polymer exemplified below but is also applicable to other polyolefins and their chemical modifications as base synthetic polymers. It is also not limited to the carbohydrates exemplified below, but may include all variations of monosaccharides, disaccharides and oligosaccharides and their derivatives. The invention is also not limited to the catalysts exemplified, but includes their derivatives and other catalysts.
EXPERIMENTAL/ EXAMPLES
The following examples are given by way of illustration and therefore should not be construed to limit the scope of the present invention.
Example 1
Poly(styrene-co-maleic anhydride) (referred to as PSMAH) containing 14-wt % maleic anhydride (PSMAH) and lactose were dried overnight in a vacuum oven at ~ 60°C. PSMAH (10g) dissolved in dry N,N'-Dimethylformamide (DMF) (125mL) was added to a stirred solution of lactose (15.44g) and 4- N,N'-dimethylaminopyridine (4 - DMAP) (250mg) in dry DMF (200mL) over a period of 30 minutes. The mole ratio of the base synthetic polymer to the carbohydrate was 1:3. The reaction mixture was stirred at 60-65°C in presence of dry nitrogen for 4 hours. The reaction mixture was precipitated in brine, filtered, washed with water several times till free of chloride, dried to obtain the product.
Example 2
Poly(styrene-co-maleic anhydride) (PSMAH) and D-glucose were dried overnight at ~60°C in a vacuum oven. PSMAH (10g) dissolved in dry N,N'-dimethylformamide (DMF) (150mL) and was added to a solution of D-glucose (7.72g) and 4- N,N'-dimethylaminopyridine (4- DMAP) (250mg) in dry DMF (150mL) over a period of 1 hour. The mole ratio of the base synthetic polymer to the carbohydrate was 1:3. The reaction mixture was stirred at 60°C for 4 h in presence of dry nitrogen. The reaction mixture was precipitated in brine, filtered, washed with water several times till free of chloride and dried to obtain the product.
Example 3
Poly(styrene-co-maleic anhydride) (PSMAH) and sucrose were dried overnight in a vacuum oven at ~60°C. PSMAH (10g) dissolved in dry N,N'-dimethylformamide (DMF) (150mL) and was added to a solution of sucrose (14.67g) and 4- N,N'-dimethylaminopyridine (4 - DMAP) (250mg) in dry DMF (150mL) over a period of 1 hour. The mole ratio of the base synthetic polymer to the carbohydrate was 1:3. The reaction mixture was stirred at 48-50°C for 18 h in presence of dry nitrogen. The reaction mixture was precipitated in brine, filtered, washed with water several times till free of chloride and then dried to obtain the product.
Example 4
Poly(styrene-co-maleic anhydride) (PSMAH) and D-glucose were dried overnight at ~60°C in a vacuum oven. PSMAH (l0g) dissolved in dry N,N'-Dimethylformamide (DMF) (150mL) and was added to a solution of D-glucose (7.72g) and 4- N,N'-dimethylaminopyridine (4 - DMAP) (250mg) in dry DMF (150mL) over a period of 1 hour. The mole ratio of the base synthetic polymer to the carbohydrate was 1:3. The reaction mixture was stirred at 47- 48°C for 18 h in presence of dry nitrogen. The reaction mixture was precipitated in brine, filtered, washed with water several times till free of chloride and dried.
Example 5
Poly(styrene-co-maleic anhydride) (PSMAH) and D-glucose were dried overnight at ~60°C in a vacuum oven. PSMAH (10g) dissolved in dry N,N'-dimethylformamide (DMF) (150mL) and was added to a solution of D-glucose (1.29g) and 4- N,N'-dimethylaminopyridine (4 - DMAP) (250mg) in dry DMF (150mL) over a period of 1 hour. The mole ratio of the base synthetic polymer to the carbohydrate was 2:1. The reaction mixture was stirred at 50°C for 18 h in presence of dry nitrogen. The reaction mixture was precipitated in brine, filtered, washed with water several times till free of chloride and dried.
Example 6
Poly(styrene-co-maleic anhydride) (PSMAH) and sucrose were dried in a vacuum oven at ~60°C. PSMAH (l0g) dissolved in dry N,N'-dimethylformamide (DMF) (150mL) and was added to a solution of sucrose (2.5g) and 4- N,N'-dimethylaminopyridine (4 - DMAP) (250mg) in dry DMF (150mL) over a period of 1 hour. The mole ratio of the base synthetic polymer to the carbohydrate was 2:1. The reaction mixture was stirred at 50°C for 18 h in presence of dry nitrogen. The reaction mixture was precipitated in brine, filtered, washed with water several times till free of chloride and dried.
Example 7
Poly(styrene-co-maleic anhydride) (PSMAH) and methyl glucoside were dried in a vacuum oven at ~60 °C. PSMAH (10g) dissolved in dry N,N'-dimethylformamide (DMF) (150mL) and was added to a solution of methyl glucoside (8.3g) and 4- N,N'-dimethylaminopyridine (4 - DMAP) (250mg) in dry DMF (150mL) over a period of 1 hour at 50-55 °C under argon. The mole ratio of the base synthetic polymer to the carbohydrate was 1:3. The reaction mixture was stirred at 50-51°C for 18 h in presence of argon. The reaction mixture was precipitated, filtered, washed with water several times till free of chloride and dried.
CLAIMS
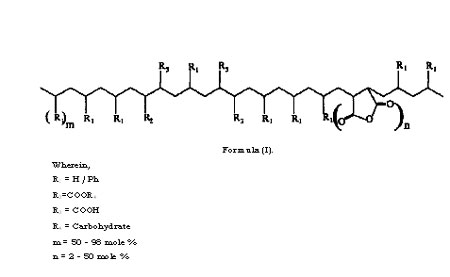
- Biodegradable synthetic polymers having a formula
- A process of the preparation of biodegradable synthetic polymers of formula (I), which comprises drying a base synthetic polymer and a carbohydrate in vacuum at a temperature ranging between 55 to 60 °C for a period of 17 to 19 hrs., preparing separately the solutions of the base synthetic polymer in dry organic solvent and a solution of a carbohydrate and a catalyst in dry organic solvent, adding the solution of the base synthetic polymer into the solution of carbohydrate and the catalyst under agitation, heating the reaction mixture to a temperature ranging between 25 to 110°C for 2-48 hours under nitrogen and agitation, cooling the reaction mixture to room temperature, precipitating the product using a solution of an inorganic salt in a non-solvent, washing the product with a solvent, till the product is free from the salt to obtain the product.
- A process as claimed in claim (2) wherein the preferred base synthetic polymer is a polyolefin, which includes polystyrene with reactive functional groups like carboxyl, hydroxyl, amino, alkene, halide, ester and anhydride.
- A process as claimed in claim (2) wherein the carbohydrates include monosaccharides, and disaccharides: both reducing and non-reducing, and oligosaccharides, wherein none of the hydroxyls are protected, and carbohydrates in which at least one but not all hydroxyls are protected.
- A process as claimed in claim (2) wherein the dry organic solvents used for the preparation of the base synthetic polymer are dry N,N'-dimethylformamide, dry pyridine, dry toluene and their mixtures.
- A process as claimed in claim (2) wherein the catalysts are pyridine, 4-dimethylaminopyridine, para-toluenesulfonic acid and carbodiimide catalysts.
- A process as claimed in claim (2) wherein the dry organic solvent used for the preparation of solution of carbohydrate and the catalyst may be dry N,N'-dimethylformamide or dry pyridine and their mixtures.
- A process as claimed in claim (2) wherein the molar ratio of base synthetic polymer to the carbohydrate may be 1:0.01 or 1:1 or 1:2 or 1:6, but preferably 1: 0.5 or more preferably 1:3.
- A process as claimed in claim (2) wherein the molar ratio of the functional groups of the base synthetic polymer and the catalyst may be 1: 0.01 upto 1:2, but preferably 1:0.14.
- A process as claimed in claim (2) wherein the non- solvent for the preparation of the salt solution may be water, methanol, ethanol, acetone and their mixtures.
- A process as claimed in claim (2) wherein the salt used for the preparation of the salt solution are sodium and potassium salts, which include sodium chloride, potassium chloride, sodium bromide, potassium bromide and their mixtures.
- A process as claimed in claim (2) wherein the solvents used for washing the product to make it free from salt are water, ethanol, methanol, acetone and their mixtures.
- Biodegradable synthetic polymers and process for preparation thereof substantially as herein described with reference to the examples and drawings accompanying this specification.
CONCLUSION
The main advantages of the present invention are :
- Development of new polymers based on synthetic polymers, especially polyolefins, by functionalizing them and linking small quantities of monosaccharides, disaccharides, and oligosaccharides in a facile manner by polymer analogous reactions.
- The basic physical properties of the modified synthetic polymers prepared as in #1 are not changed substantially by linking a few carbohydrate molecules, thereby extending their applications for legally and socially acceptable disposable products.
- The process is economic and simple.
- The products are environment friendly due to their biodegradability and substantially as herein described with reference to the examples accompanying this specification.
Biomineralization of the Sugar-linked Poly(styrene maleic anhydride)
INTRODUCTION
The term biodegradation involves bioassimilation and biomineralization. Bioassimilation involves ingestion of polymer fragments by microorganisms. Biomineralization is a process in which the organic material or the polymer is completely broken down and converted to carbon dioxide, methane, water, salts, nitrogen and biomass, so that all the elements from the polymer re-enter the natural geochemical and microbial cycles. There are several standards to assess the biodegradation of polymers like the ISO 472:1988, ASTM (D20.96 proposal) and DIN 103.2. However these methods fail to classify them in terms of the rates of biodegradation of the polymer and also do not consider their ultimate fate. The test methods included in the OECD guidelines, which are considered to be appropriate tests, include the modified Strum test, the modified MITI test and the closed bottle test (OECD 301B, C, D). All the tests are carried out under aerobic conditions in aqueous medium wherein, the polymer provides the sole source of carbon for the microorganisms. Any polymer, which degrades to 60% of the total carbon content, is considered to be readily degradable.
The extraordinary abundance of microorganisms available consists of both autotrophic and heterotrophic bacteria, which share a symbiotic relationship. The autotrophs are capable of utilizing carbon dioxide from the atmosphere directly, whereas the heterotrophs depend on the autotrophs for their carbon source. Hence the carbon dioxide evolved from the degradation of the polymer thus enters the geochemical cycle.
Principle of the Organisation for Economic Co-operation and Development (OECD) method for determination of biodegradation by biomineralization is based on the measuring the amount of carbon dioxide evolved from the sample under consideration, in which the sample is the sole source of carbon for the microorganisms.
EXPERIMENTAL
Experimental set-up
Carbon dioxide, hydrocarbon and moisture- free air was distributed into five one litre capacity reactor flasks. The reactor flasks were constantly stirred by magnetic stirring bars. All the flasks contained 250 mL of the minimal medium and the inoculum. Flask-1 contained only the minimal medium without any carbon source. In addition to this, Flask-2 contained unmodified poly(styrene maleic anhydride) (PSMAH), Flask-3 contained glucose- linked PSMAH, Flask-4 contained sucrose- linked PSMAH and Flask-5 contained lactose- linked PSMAH. The evolved carbon dioxide was trapped in barium hydroxide traps (0.0125M solution) (three traps arranged consecutively for each flask). The first trap of each set/ flask was removed for titration and the second trap was shifted in place of the first trap, the third was shifted to the place of the second one and a new trap was placed in place of the third trap. 25 mL of barium hydroxide solution withdrawn from the middle portion of each trap was titrated with 0.05M HC1 solution using phenolphthalein indicator. Standardization of ~ 0.05M HC1 was carried out using ~ 0.05M NaOH solution. NaOH solution (~0.05 M) was standardized using potassium hydrogen phthalate solution.
Flask 1: Minimal medium + inoculum
Flask 2: Minimal medium + inoculum + PSMAH
Flask 3: Minimal medium + inoculum + glucose- linked PSMAH
Flask 4: Minimal medium + inoculum + sucrose- linked PSMAH
Flask 5: Minimal medium + inoculum + lactose- linked PSMAH
All traps contain ~ 0.01255M Barium hydroxide solution
Composition of minimal medium for 1 litre solution
Potassium dihydrogen phosphate (KH2PO4) : 85 mg
Dipotassium hydrogen phosphate (K2HPO4) : 217 mg
Disodium hydrogen phosphate (Na2HPO4.2H2O) : 334 mg
Ammonium chloride (NH4C1) : 5 mg
Calcium chloride (CaCl2.2H2O) : 36.4 mg
Magnesium sulfate (MgSO4.7H2O) : 22.5 mg
Ferric chloride (FeCl3.6H2O) : 0.25 mg
pH: 7.4 ±0.2
Solutions for the titration are as follows
5M Sodium hydroxide solution: sodium hydroxide (200g) dissolved in distilled water to make 1 litre solution.
0.05M Barium hydroxide solution: barium hydroxide (1.71g) dissolved in distilled water to make a 500mL solution.
0.05M Hydrochloric acid solution: hydrochloric acid (4.5 mL) diluted upto 1 litre with distilled water.
0.05M Sodium hydroxide solution: sodium hydroxide (1g) dissolved in distilled water to make 500mL solution.
0.0125M Barium hydroxide solution: barium hydroxide (3.94g) dissolved in distilled water to make 1 litre solution and then filtered to remove insoluble particles.
Potassium hydrogen phthalate solution: accurately weighed potassium hydrogen phthalate (0.2 g) dissolved in 50 mL of water for standardization of ~ 0.05M sodium hydroxide solution.
Preparation of the inoculum
Pure culture viz. Serratia marscecens was used in place of the activated sludge used in the OECD method. The culture was transferred from the slant to 10mL of nutrient broth on day one. On day two, the 10mL-inoculated broth was transferred to 100mL of nutrient broth. This 100mL broth was centrifuged on day three at ~4000rpm for 15 minutes. The cells were washed with saline, centrifuged again, suspended in l0mL saline and used as an inoculum. 1.5 mL of the bacterial suspension was added to each flask.
RESULTS AND DISCUSSIONS
The set- up was standardized using different concentrations of glucose (100 mg/L -1400 mg/L in terms of total organic carbon). The optimum concentration was found to be 200 mg/L of carbon based on the amount of mg of CO2 evolved and percent biodegradation of glucose standard. The results were reproducible and the tests were extended to the test polymers. The starting polymer, viz. poly(styrene maleic anhydride) contained 85% carbon, whereas the sugar- linked poly(styrene maleic anhydride) polymers contained 80% carbon, according to elemental analysis. The amounts of the polymers were chosen such that the concentration of the total organic carbon was 200 mg/L in the minimal medium.
Formulas used for the calculation of mg of CO2 evolved & % Biodegradation.
mg of CO2 evolved =Normally of HCL/2 Ã- mL of HCL titrated x mol. wt. of CO2
% Biodegradation =
The amount of carbon was determined from the percentage of carbon in the polymer and the weight of the polymer taken.
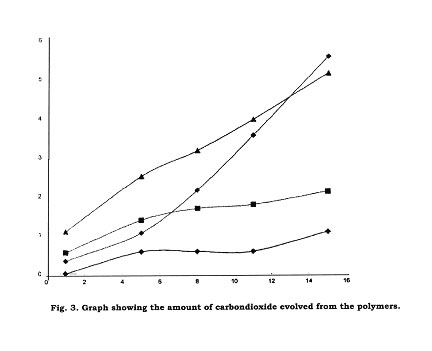
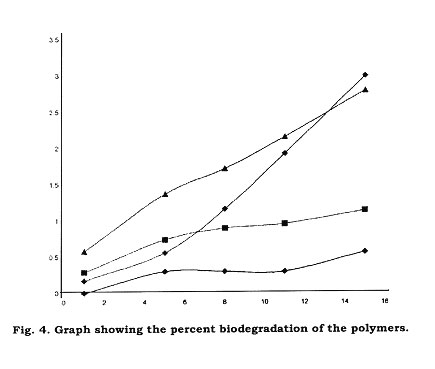
Biotechnology: an Enabling Technology
Modem biotechnology is a powerful and versatile tool which can compete with chemical and physical means of reducing energy and material consumption and minimizing the generation of waste and emissions.
Biotechnology is an increasingly powerful tool for achieving industrial sustainability. Biotechnology offers one important path to cleaner products and processes. Biotechnology per se is not necessarily clean, just as chemical of industrial processes, both because it is so versatile and because its power continues to grow. Living systems manage their chemistry rather more efficiently than chemical plants and their wastes are generally recyclable and biodegradable. This, along with our increasing ability to manipulate biological materials and processes, strongly points to a significant impact on the future of manufacturing industry.
It can help reduce atmospheric pollutants, waste, and energy and raw materials use. Biotechnology can help lower the production of greenhouse gases and acid rain. Using biorenewable feedstocks instead of fossil fuels can slow atmospheric build- up of carbon dioxide and help alleviate other problems of atmospheric pollution.
BIOTECHNOLOGY AND CO2 EMISSIONS
Fossil carbon is the single most important raw material for energy generation and chemicals manufacture, but its use often produces carbon dioxide (CO2), an important greenhouse gas. Any means of reducing fossil carbon consumption, either by improving energy efficiency or substituting alternative resources, directly results in lowered CO2 production and reduces global warming. Biomass, which yields only as much CO2 as it takes up during growth, can serve as a chemical feedstock or a source of energy.
Biomass can be consumed directly (incinerated) to produce energy. It can also be converted into a wide range of chemicals and liquid fuels. In energy terms, annual production of biomass is some five times global energy consumption but currently provides only 1% of commercial energy. At present, however, it is much more expensive than fossil fuels and has penetrated the market only where governments have effectively subsidized its use.
Bioethanol is a CO2-neutral alternative liquid transportation fuel. As new technologies and more efficient separation techniques are developed, bioethanol will compete on cost with gasoline. Over the next two decades, US ethanol production from lignocellulosic waste could reach 470 million tons a year, the equivalent in energy terms of present gasoline consumption.
Substances made from renewable raw materials can now compete with the chemical alternatives.
A wide range of chemicals and structural materials can be made from biological raw materials (biomass), including biodegradable plastics, biopolymers and biopesticides, and novel fibers and timbers. As it grows, biomass consumes CO2 so that, unless fossil fuel is used, there is a zero net contribution to atmospheric greenhouse gases.
In the past, cheap raw materials based on fossil fuels underpinned the rapid growth of petrochemical alternatives, but environmental considerations are directing renewed attention to bioresources. Thus, while chemists have long worked to duplicate plant materials, it may now be advantageous, from a global environmental perspective, to return to starches, cellulose, vegetable oils and proteins as potential alternative raw materials for industrial production.
THE SOYA BEAN: AN IMPORTANT RENEWABLE RESOURCE
The soya bean has long been used to develop products ranging from food and diesel fuels to polymers, fabric softeners, solvents, adhesives, linoleum, rubber substitutes, printing inks, and plastics. Recent advances in recombinant genetic biotechnology have made it possible to alter the lipid composition of soya beans to increase the variety of biohydrocarbons available for industrial applications. Amides, esters and acetates of biohydrocarbons are currently used as plasticizers, blocking/slip agents and mold-release agents for synthetic polymers. Biohydrocarbons linked to amines, alcohols, phosphates and sulfur groups are used as fabric softeners, surfactants, emulsifiers, corrosion inhibitors, anti-static agents, hair conditioners, ink carriers, biodegradable solvents, cosmetic bases and perfumes. In combination with aluminum and magnesium, the soya bean is used to produce greases and marine lubricating materials.
Carbon dioxide is a virtually unlimited raw material with many uses. Carbon dioxide is the fundamental source of renewable organic compounds. Microalgae can be used to produce lipids, biodiesel fuels and the antioxidant vitamin beta-carotene. Though algae-derived biodiesel has only been produced at laboratory scale and only 10 tons of beta-carotene are produced world-wide, carbon dioxide use could easily be expanded by orders of magnitude if new or improved processes could be developed.
Biotechnology is gaining ground for the production of commodity chemicals. Commodity chemical production, where both chemical and biotechnological approaches are used, is a pioneering field for biotechnology. In the United States, commodity chemicals currently derived largely from plant matter include ethanol (3.8 million tons a year), cellulose esters and ethers (0.5 million tons a year), sorbitol (0.19 million tons a year) and citric acid (0.16 million tons a year). New processes and renewable resources for other commodity chemicals such as succinic acid and ethylene glycol, are in the pilot stage in government-sponsored programs in partnerships with private enterprise. These are currently made almost exclusively from petrochemical feedstocks.
Genetically engineered microorganisms can be used to replace toxic industrial processes. Because microorganisms can synthesize a wide range of compounds, using carbohydrates as the sole energy and carbon sources, companies are exploiting them to obtain specialty chemicals. In ancient times, indigo, which is used today for dyeing denim, was obtained from plants; today, it is synthesized from toxic chemicals, including aniline, formaldehyde, and sodium cyanide. Through genetic engineering, an indigo produced from a microorganism will soon be on the market.
CHEMICALS FROM BIOLOGICAL FEEDSTOCKS
It is no longer necessary to start with a barrel of oil to produce chemicals. Corn, beets, rice - even potatoes - make great feedstocks. The transformation of sugars into alcohol by microorganisms has been known for a very long time. But only since the advent of genetic engineering is it feasible to think about harnessing the sophistication of biological systems to create molecules that are difficult to synthesize by traditional chemical methods.
For example, compared to traditional polyester (2GT), the polymer polytrimethylene terephthalate (3GT) has improved properties. Yet commercialization has been slow because of the high cost of making trimethylene glycol (3G), one of 3GT's monomers.
The secret to producing 3G can be found in the cellular machinery of certain unrelated microorganisms. Some naturally occurring yeasts convert sugar to glycerol, while a few bacteria can change glycerol to 3G. The problem is that no single natural organism has been able to do both.
Through recombinant DNA technology, an alliance of scientists from DuPont and Genencor International has created a single microorganism with all the enzymes required to turn sugar into 3G. This breakthrough is opening the door to low-cost, environmentally sound, large-scale production of 3G. The eventual cost of 3G by this process is expected to approach that of ethylene glycol (2G).
The 3G fermentation process requires no heavy metals, petroleum or toxic chemicals. In fact, the primary material comes from agriculture - glucose from cornstarch. Rather than releasing carbon dioxide to the atmosphere, the process actually captures it because, although microbes do produce CO2 during fermentation, corn absorbs CO2 as it grows. All liquid effluent is easily and harmlessly biodegradable. What's more, 3GT can readily undergo methanolysis, a process that reduces polyesters to their original monomers. Post-consumer polyesters can thus be repolymerized and recycled indefinitely.
Biocatalysts, notably enzymes, offer an extremely promising source of improved processes.
Biotechnology enables the rapid and controlled production of biological catalysts -living organisms and their catalytically active constituents - particularly enzymes. Because they are more specific and more selective than their non-biological counterparts, biological catalysts yield fewer by-products (specificity) and start with less purified raw materials (selectivity). They are also amenable to continuous improvement. Despite their advantages, they still present problems for industrial applications, as they may be fragile, require large amounts of water, and are costly. Many of these problems have been addressed and overcome using new bioreactor designs and improved catalysts.
Biotechnology can be used in all phases of an industrial process. In integrated bioprocessing, biocatalyst activity can simplify the overall process by reducing the number of stages. For example, cellulose can simultaneously be broken down and fermented to glucose with the use of enzymes. Yeast is used to ferment the glucose to ethanol in order to prevent feedback inhibition of the cellulose hydrolysis. Ethanol, in turn, depresses the fermentation rate and is therefore extracted using a water-immiscible solvent, oleyl alcohol. All these reactions take place in a single reactor vessel.
It is essential to determine whether biotechnology-based processes are cleaner than traditional ones ...
Clearly, biotechnology-based manufacturing is gaining ground and has a great deal to offer. Enzyme-based processes operate at lower temperatures and produce fewer and less toxic by-products and emissions than conventional chemical processes. Use of biotechnology has already succeeded in reducing energy use in certain industrial processes. However, the key issue is whether, overall, biotechnology-based processes are significantly cleaner than traditional chemical processes.
... and life cycle assessment is currently the best way to do so. There are a number of "tools" for measuring the effect of technology on the environment. Of these, the best is life cycle assessment (LCA), which is a way of evaluating the environmental impact of alternative products and processes in terms of their use of energy and materials. LCA takes into account the entire life cycle of a process or product from the "cradle to the grave".
Life cycle assessments of biotechnological operations confirm their superior cleanliness. Because it is global and holistic, that is, because it can cover everything from obtaining the raw materials to disposing of the product at the end of its useful life, whatever the geographic location, this type of analysis offers a way to:
- decide whether a process, product or service is in fact reducing the environmental load or merely transferring it upstream to resource suppliers or downstream to treatment or disposal;
- determine where in a process the most severe environmental impact is felt;
- make quantitative comparisons of alternative process options and of competing technologies.
Although LCA was first developed over two decades ago, it has been little used so far for bioprocesses and products, partly because of biotechnology's relatively late arrival on the industrial scene and partly because it raises particular methodological problems. However, where it has been used, it has generally confirmed that biotechnological operations are cleaner and more economical.
The attractiveness of LCA lies in its use of the life cycle concept for products/systems and the possibility of objective or fair comparisons of industrial systems. It defines clearly the scope and goals of a specific evaluation and sets its boundaries (where, in other words, the assessment starts and stops); it collects data on the relevant inputs and outputs (use of energy and resources, emissions into the environment); and it assesses the impact of the various parameters on the ecological system and on human health.
LIFE CYCLE ASSESSMENT OF PROTEASES
Proteases, which remove protein impurities, are essential components of modern detergents. Because of their catalytic effect, low concentrations (0.1-1.0%) are used. Similar washing performance cannot be achieved by substituting other substances or by raising the washing temperature.
An LCA was made to compare the production processes for proteases obtained from a traditional microorganism and one from a genetically modified organism in order to determine the pollution linked to enzyme production and to reveal any weaknesses in the production process.
The assessment only covered enzyme production, not the whole life cycle of detergent proteases. It included all processes from raw materials to the finished granulated product as well as transport of raw materials from the individual manufacturers to the enzyme producers.
To produce a quantity of enzyme with an equivalent washing performance from the recombinant organism required 34% less raw material. The change from conventional production to production using the new organism reduced the demand for process energy by 60%. In terms of the manufacturer's total annual requirements, this energy saving corresponds to the annual primary energy consumption for laundry purposes of about 170,000 households. Application of the new enzyme produced with genetically modified organisms made it possible to reduce annual emissions by approximately 170 tons of carbon and 190 tons of sulfur dioxide. Atmospheric emissions were assessed in terms of impact on global warming, development of acid rain, and smog formation. Aquatic emissions were assessed according to the yield of nutrients in water and related oxygen consumption. The assessment clearly confirmed the claim that the use of the new organism reduced consumption of energy, resources and emissions by a factor of 3 to 4.
A steady stream of biotechnological innovations offers new opportunities for industry. In sum, biotechnology can offer a wide-ranging set of tools for improving large-scale fermentations to produce chemicals such as ethanol, at one end of the spectrum, to using minute parts of biological molecules as sensors in analytical devices, at the other. New products from industrial biotechnology include more functional products, often at approximately the same price as traditional ones, such as biodegradable polymers, optically active chemicals, and enzymes for use in detergents and feeds. The ability to alter the characteristics of living organisms and their constituent parts is now such that many industries are actively investigating the opportunities and many new possibilities are emerging from R&D.
Starch Based Biodegradable Plastics
INTRODUCTION
Technology transfer and commercialization of university research and industry-university research programs leading to a commercial venture is a difficult and sometimes elusive pursuit. The typical technology transfer approach practiced by Universities involves licensing of the technology to a company. This is a passive approach and many promising technologies have fallen by the wayside because (a) it does not meet the financial or market volume of large corporations who typically license university research (b) the lack of applied research, engineering and economic demonstration data that is essential for the company to make an investment decision - bringing the fundamental university technology to "investment-grade" (c) the market risk and needed expertise to bring the technology to fruition - no committed technical/business champion. These problems are magnified if the University research involves a completely new technology field and a new market. Biodegradable plastics from agricultural feedstocks falls into the new technology, new market category and is the subject of this paper.
MBI International is a not-for-profit Applied Research & Development Institute set up by the State of Michigan to identify, develop, and commercialize biobased product technologies. At MBI we practiced a pro-active technology commercialization model that ultimately results in effective technology transfer of research to a start-up or joint venture company through MBI and its business subsidiary Grand River Technologies (GRT). Ofcourse, the technology, has to meet certain specified business and market criteria before a business can be established. I have been involved in the development and commercialization of four bio-based product technologies using the above model. Thetechnologies are:
- Poly(lactic acid) biodegradable plastics: Engineering, scale-up, and applications research for poly(lactic acid) biodegradable plastics technology were conducted at the university and at the Institute with collaboration and support of Cargill Inc., one of the worlds largest agribusiness. Cargill is currently commercializing this technology world-wide, and recently announced the creation of a joint venture company with Dow Chemical, Cargill-Dow LLC for the same.
- Modified Starch Biodegradable Thermoplastics: EverCorn, Inc., a joint venture between Grand River Technologies (GRT), the business subsidiary of Michigan Biotechnology Technology, and Japan Corn Starch Company, is involved in design, engineering & manufacture of thermoplastic, modified-starches which have water repellent properties, mechanical strength, and good processability, while being fully biodegradable in appropriate disposal systems like composting. A two year $2.0 MM R&D phase was completed in July 1995. Pilot scale operations are in place to sample customers with thousands pound quantities of resin.
- Starch-Polyester Biodegradable Plastics: BioPlastics Inc., a start-up company, is involved in manufacture of starch-polycaprolactone resins that is designed to have water resistance, good strength properties and ease of processability, while being fully biodegradable under composting conditions. The technology was developed by graduate students at the University and in-licensed through MBI to BioPlastics (four patents). The company launched in February 1995 and capitalized with $500,000. The initial target market is compost bags (lawn & leaf bags), retail & merchandise bags.
- Sugar based BioAdhesives: Lions Adhesives Inc., a start-up company, is commercialization a portfolio of environmentally friendly packaging and wood adhesives based on annually renewable resources. The adhesives are designed to be water resistant, have good adhesive bond strength, high application speed and machine stability. They are targeted to be non-interfering in recycling operations, and biodegradable in appropriate infrastructures. The company was launched in 1997, and capitalized with $600,000 from a private investor.
The Poly(lactic acid) technology development and commercialization represents the standard approach wherein the University and the Institute performs R&D under contract with the company (Cargill, in this instance). The other three technologies represents the pro-active commercialization model, wherein the technology is being commercialized through the creation of a start-up company or joint venture with a large corporation.
In this paper, I will discuss the elements of a technology commercialization process model, and its use in commercializing Starch-based biodegradable plastics.
TECHNOLOGY COMMERCIALIZATION MODEL
It recognizes that the only true measure of successful technology transfer is market acceptance of the technology resulting in a profitable business. The first step in the process is the generation of an idea or an invention by University faculty, or researchers at non-profit Institutes and National laboratories. Next, the technical feasibility and proof-of-concept of the new idea is established. At this stage intellectual property is being created, and needs to be protected by patents. Unfortunately, many researchers and faculty seek publication of results before protecting the intellectual property and considerably diminish the value and commercialization potential of the technology. The second step in the commercialization process involves assessment of the technology for its business and market potential. This is best done by persons with commercial or business expertise in that technology area. Typically, such expertise does not reside at the University or research institutes. Therefore, the standard approach is to seek the opinion and support of a company working in that technology area to further develop the technology. If the company is interested, it licenses the technology (if patents have been filed) and evaluates the commercialization of the technology using its own criteria and measures. If the technology is not protected, then the company is much less likely to pursue commercialization. This is because of the fear that another company can easily enter the same market after they have spent considerable money and time on developing the market and technology,. As discussed in the Introductory section, companies may, also, choose not to commercialize the technology because it does not meet their market volumes or hurdle rate or business reasons. Typically, at this stage technology commercialization efforts die. However, the technology may be perfectly viable for a start-up small business operation. Therefore, there is need for a business development infrastructure/expertise to assess the business and market potential of the technology. If there is limited or no business/market potential for the technology, then the technology goes back to the first step to be modified to address the identified technical or market issue. Cost is the single most important issue that drives commercialization forward. Preliminary costing, especially materials costs should be calculated to establish that material costs are in the target range of the materials to be substituted. If the potential is high, then the project moves on to step 3. The technology is refined, product specifications and process parameters developed. Preliminary engineering economics is completed. Detailed market analysis, product sampling and demonstration is conducted. A Business Plan is developed. A start-up business can be established to commercialize the technology if the initial capital requirements for starting the business is around $2-5 million. However, if the capital requirements are much higher, and/or the process is complex, then a joint venture or out-licensing the technology to an established corporation doing business in the technology area is appropriate. Out-licensing at this stage of the technology development as opposed to after step 1 significantly enhances the success of technology commercialization and adds considerable value to the technology.
APPLICATION OF TECHNOLOGY COMMERCIALIZATION MODEL
Step 1 of the commercialization model involves generation of the technology idea, and creation of a research project to generate the data necessary to establish proof-of-concept for the technology. This is standard procedure at Universities, research institutes, National labs etc. As discussed in the earlier section, step 2 requires a business development infrastructure or expertise to move the technology forward towards commercialization. MBI International, a non-profit Institute, provides the business infrastructure through its Biobusiness Incubator, and its for-profit subsidiary Grand River Technologies (GRT). The incubator facility allows a start-up company to locate there and develop the business before graduating to full-scale commercial operations. Such business incubators and infrastructures are developing near Universities to provide the link to the next steps in commercializing technology. It follows the technology commercialization model illustrated earlier.
Starch-based Biodegradable Plastics - Commercialization Case Studies
We initiated four R&D projects in the general area of Biodegradable Plastics from agricultural feedstocks with the ultimate goal of commercializing the technologies that would result from it.
Biodegradable Plastics Rationale. New environmental regulations, societal concerns, and a growing environmental awareness throughout the world have triggered the search for new products and processes that are compatible with the environment. Thus, new products have to be designed and engineered from cradle to grave incorporating a holistic "life cycle thinking" approach. The impact of raw material resources used in the manufacture of a product and the ultimate fate (disposal) of the product when it enters the waste stream have to be factored into the design of the product. The use of annually renewable resources and the biodegradability or recyclability of the product are becoming important design criteria. This has opened up new market opportunities for developing biodegradable products. Designing and engineering new materials that are biodegradable and ensuring that they end up in an appropriate disposal system is environmentally and ecologically sound. For example, by composting our biodegradable plastic and paper waste along with other "organic" compostable materials like yard, food, and agricultural wastes, we can generate much-needed carbon-rich compost (humic material). Compost amended soil has beneficial effects by increasing soil organic carbon, increasing water and nutrient retention, reducing chemical inputs, and suppressing plant disease. Composting infrastructures, so important for the use and disposal of biodegradable plastics, are growing in the U.S. and are in part being regulatory driven at the state level.
Poly(Lactic Acid) based Biodegradable Plastics. Hydrolysis of corn starch or cellulosic materials yields simple sugars that can be readily fermented into lactic acid. L-Lactic acid is produced by the bacterial fermentation of corn sugar (D-glucose): C6H12O6 = 2C3H6O3, DGo(25oC) = -136kJ/mol. Purac Biochem BV (division of CSM) currently produces an estimated 80% of the worlds lactic acid. Cargill and Purac have (5/96) a 50-50 joint venture to build and operate a 70 million lb/yr lactic acid facility in the US; the current US consumption of lactic acid is 55 million lb/yr.
The conventional route to high molecular weight PLA is through the dilactone of lactic acid. Polylactide polymers are primarily used in biomedical applications. At Michigan State University and MBI, we initiated a R&D project on Design and Engineering of polylactide (PLA) polymers for industrial applications. Fundamental R&D and engineering was carried out to establish proof-of-concept of PLA polymers for industrial applications - Step 1 of the Technology Commercialization model. The project was supported by Cargill Inc., and done in collaboration with them on a contract basis. In this case, the technology commercialization followed the typical, standard, approach wherein a large corporation with interests in the area took over the technology commercialization efforts. Therefore, in this case the model is not appicable, especially steps 2 to 4.
Starch Ester based Biodegradable Thermoplastics. R&D was conducted at MBI to develop a family of biodegradable thermoplastic starch esters for injection molded products and coatings. .Modification of the starch -OH groups by esterification chemistry to form starch esters of appropriate degree of substitution (1.5 to 3.0 ds) imparts thermoplasticity - can be processed and shaped like current plastic products. Unmodified starch shows no plastic behavior and thermally degrade around 260 0C. Plasticizers like glycerol triacetate and diethyl succinate are completely miscible with starch esters and can be used to improve processability. Water resistance of the starch esters is greatly improved over the unmodified starch. The technology is protected by several patents. Based on preliminary economics and process engineering studies, it was established that capital investment costs would be high, and the process complex. Therefore, it would be difficult for a start-up company to initiate commercialization of the technology. A joint venture with a large starch processing company would be needed to commercialize this technology. A joint venture company (EverCorn Inc.), was established between MBI/GRT and Japan Corn Starch (one of Japan's leading starch based industrial products company) to commercialize this technology. Appropriately formulated starch esters with plasticizers and other additives provide resin compositions that can be used to make injection molded products and for direct lamination onto Kraft paper. These new, modified-starches have water repellent properties, mechanical strength, and good processability, while being fully biodegradable in appropriate disposal systems like composting. A two year $2.0 MM R&D phase was completed in July 1995. Pilot scale operations are in place to sample customers with thousands pound quantities of resin and a full-scale operational plant is under development.
Starch-Poly(e-caprolactone) (PCL) alloys - We developed a new technology at Michigan State University to produce biodegradable thermoplastic starch-polyester alloys for film applications. This technology involves reactive extrusion processing of plasticized starch with modified PCL in a twin screw co-rotating extruder with modified screw elements. By controlling the rheology in the extruder, one can obtain a morphology in which the plastic starch is dispersed in a continuous PCL matrix phase. Good adhesion and compatibilization is promoted between the plastic-starch phase and the modified PCL phase to obtain enhanced mechanical properties. Some of the advantage of using plasticized starch instead of granular starch are:
- smaller domain size is possible by controlling rheological characteristics
- improved strength and processing characteristics
- reduced macroscopic dimensions in certain applications, like film thickness
All of the operations can be performed in the extruder, thereby eliminating the use of solvent, reducing the number of steps to making the final resin, and simplifying the process operations. On a Life-cycle basis, we not only engineered a biodegradable product, but also reduced waste generation, energy consumption, and conserved resources.
This technology was ideal for a start-up business because of the relatively small capital requirements ($2-3 million) and the simplicity of the process - essentially a compounding operation. The technology is covered by four patents. A strong intellectual property position is an important element for successful business operations.
A start-up company, BioPlastics Inc., was formed under the MBI/GRT umbrella and located at the MBI incubator facility to commercialize the technology. The technology was licensed by MSU to MBI. BioPlastics Inc., is manufacturing and sampling customers with this new starch-PCL resin that is being marketed under the name "ENVAR" for film applications like compost bags, trash and retail carry-out bags etc. Seed capital for the company came from a consortium of State Corn Grower Associations, the State of Michigan, and USDA SBIR (Small Business Innovation Research) programs.
This technology would have languished on the shelf if the standard practice of looking for a potential licensee from a large corporation would have been followed. There are several reasons for this (note comments in introductory section). The major one is that this represents a new technology in a new market, and not improvements to an existing technology or new technology to improve an existing market.
BioAdhesives. Lions Adhesives was founded in 1996 to develop and market a family of VOC(volatile organic content)-free waterborne adhesives that are biodegradable and non-interfering in repulping operations of paper and paperboard products. The technology involves incorporating "designer sugar molecules", derived from annually renewable resources such as corn. The company is developing patents [[14],[15]] and proprietary know-how around these new technologies and positioning itself to become a $20 MM company in five years. It will explore the licensing of its patented technology and proprietary know-how as a part of its commercialization strategy
The commercialization of this technology followed a slightly different path than in the technology commercialization model. The generation of the Bioadhesives business or product (repulpable and biodegradable sugar-based waterborne adhesives) idea (Step 1 of the model) was followed by an analysis of the market and business potential for the technology which included, of course, product cost analysis - Step 2 of the commercialization model. Thus, the business analysis preceded detailed R&D work on the project with the establishment of intellectual property positions as envisioned in Step 1of the model. However, preliminary scoping research to show proof-of-concept was done prior to business and market analysis. This modified approach, blurs the boundary between Step 1 and Step 2 of our model. Basically it involves:
- Idea generation, and "scoping research" to show technical feasibility (step 1 of the model),
- Technology assessment, business and market analysis (Step 2),
- Detailed R&D, proof-of-concept and intellectual property creation (Step 1).
An investor group, Lions Investments, put in the initial seed financing of $600,000 for the company, and GRT provided the costs for office, laboratory, and pilot plant space including equipment usage.
CONCLUSION
The typical technology transfer and commercialization process involves licensing a patented technology to an interested company. This passive approach fails many times because the technology does not meet the company's business and/or product portfolio criteria. However, the technology may be perfectly viable from a technical and business sense, especially for start-up business. In many cases, more detailed business, market, and engineering analysis is needed to bring the technology to "investment grade", so a company can make an informed decision to pursue commercialization.
An integrated step by step technology commercialization process model is presented that seeks to add value to the technology by integrating business and market analysis, engineering, pilot scale-up, demonstration trials, and a good operational business plan. I believe that following such a process model would significantly enhance the commercialization of technologies - especially in areas like new industrial products from agricultural feedstocks.
Four case studies of technology commercialization using agricultural feedstocks have been discussed that encompasses out-licensing, joint venture, and start-up business creation.
Making Packaging Greener - Biodegradable Plastics
Our whole world seems to be wrapped in plastic. Almost every product we buy, most of the food we eat and many of the liquids we drink come encased in plastic. In Australia around 1 million tonnes of plastic materials are produced each year and a further 587,000 tonnes are imported. Packaging is the largest market for plastics, accounting for over a third of the consumption of raw plastic materials - Australians use 6 billion plastic bags every year!
Plastic packaging provides excellent protection for the product, it is cheap to manufacture and seems to last forever. Lasting forever, however, is proving to be a major environmental problem. Another problem is that traditional plastics are manufactured from non-renewable resources - oil, coal and natural gas.
PLASTICS THAT BREAK DOWN
In an effort to overcome these shortcomings, biochemical researchers and engineers have long been seeking to develop biodegradable plastics that are made from renewable resources, such as plants.
The term biodegradable means that a substance is able to be broken down into simpler substances by the activities of living organisms, and therefore is unlikely to persist in the environment. There are many different standards used to measure biodegradability, with each country having its own. The requirements range from 90 per cent to 60 per cent decomposition of the product within 60 to 180 days of being placed in a standard composting environment.
The reason traditional plastics are not biodegradable is because their long polymer molecules are too large and too tightly bonded together to be broken apart and assimilated by decomposer organisms. However, plastics based on natural plant polymers derived from wheat or corn starch have molecules that are readily attacked and broken down by microbes.
PLASTICS CAN BE PRODUCED FROM STARCH
Starch is a natural polymer. It is a white, granular carbohydrate produced by plants during photosynthesis and it serves as the plant's energy store. Cereal plants and tubers normally contain starch in large proportions. Starch can be processed directly into a bioplastic but, because it is soluble in water, articles made from starch will swell and deform when exposed to moisture, limiting its use. This problem can be overcome by modifying the starch into a different polymer. First, starch is harvested from corn, wheat or potatoes, then microorganisms transform it into lactic acid, a monomer. Finally, the lactic acid is chemically treated to cause the molecules of lactic acid to link up into long chains or polymers, which bond together to form a plastic called polylactide (PLA).
PLA can be used for products such as plant pots and disposable nappies. It has been commercially available since 1990, and certain blends have proved successful in medical implants, sutures and drug delivery systems because of their capacity to dissolve away over time. However, because PLA is significantly more expensive than conventional plastics it has failed to win widespread consumer acceptance.
PLASTICS CAN ALSO BE PRODUCED BY BACTERIA
Another way of making biodegradable polymers involves getting bacteria to produce granules of a plastic called polyhydroxyalkanoate (PHA) inside their cells. Bacteria are simply grown in culture, and the plastic is then harvested. Going one step further, scientists have taken genes from this kind of bacteria and stitched them into corn plants, which then manufacture the plastic in their own cells.
WHAT'S THE COST?
Unfortunately, as with PLA, PHA is significantly more expensive to produce and, as yet, it is not having any success in replacing the widespread use of traditional petrochemical plastics.
Indeed, biodegradable plastic products currently on the market are from 2 to 10 times more expensive than traditional plastics. But environmentalists argue that the cheaper price of traditional plastics does not reflect their true cost when their full impact is considered. For example, when we buy a plastic bag we don't pay for its collection and waste disposal after we use it. If we added up these sorts of associated costs, traditional plastics would cost more and biodegradable plastics might be more competitive.
BIODEGRADABLE AND AFFORDABLE
If cost is a major barrier to the uptake of biodegradable plastics, then the solution lies in investigating low-cost options to produce them. In Australia, the Cooperative Research Centre (CRC) for International Food Manufacture and Packaging Science is looking at ways of using basic starch, which is cheap to produce, in a variety of blends with other more expensive biodegradable polymers to produce a variety of flexible and rigid plastics. These are being made into 'film' and 'injection moulded' products such as plastic wrapping, shopping bags, bread bags, mulch films and plant pots.
MULCH FILM FROM BIODEGRADABLE PLASTICS
The CRC has developed a mulch film for farmers. Mulch films are laid over the ground around crops, to control weed growth and retain moisture. Normally, farmers use polyethylene black plastic that is pulled up after harvest and trucked away to a landfill (taking with it topsoil humus that sticks to it). However, field trials using the biodegradable mulch film on tomato and capsicum crops have shown it performs just as well as polyethylene film but can simply be ploughed into the ground after harvest. It's easier, cheaper and it enriches the soil with carbon.
POTS YOU CAN PLANT
Another biodegradable plastic product is a plant pot produced by injection moulding. Gardeners and farmers can place potted plants directly into the ground, and forget them. The pots will break down to carbon dioxide and water, eliminating double handling and recycling of conventional plastic containers.
DIFFERENT POLYMER BLENDS FOR DIFFERENT PRODUCTS
Depending on the application, scientists can alter polymer mixtures to enhance the properties of the final product. For example, an almost pure starch product will dissolve upon contact with water and then biodegrade rapidly. By blending quantities of other biodegradable plastics into the starch, scientists can make a waterproof product that degrades within 4 weeks after it has been buried in the soil or composted.
LANDFILL SITES AREN'T COMPOST HEAPS
To maximise the benefit of the new bioplastics we'll have to modify the way we throw away our garbage - to simply substitute new plastics for old won't be saving space in our landfills.
Although there is a popular misconception that biodegradable materials break down in landfill sites, they don't. Rubbish deposited in landfill is compressed and sealed under tonnes of soil. This minimises oxygen and moisture, which are essential requirements for microbial decomposition. For biodegradable plastics to effectively decompose they need to be treated like compost.
COMPOSTING THE PACKAGING WITH ITS CONTENTS
Compost may be the key to maximising the real environmental benefit of biodegradable plastics. One of the big impediments to composting our organic waste is that it is so mixed up with non-degradable plastic packaging that it is uneconomic to separate them. Consequently, the entire mixed waste-stream ends up in landfill. Organic waste makes up almost half the components of landfill in Australia.
By ensuring that biodegradable plastics are used to package all our organic produce, it may well be possible in the near future to set up large-scale composting lines in which packaging and the material it contains can be composted as one. The resulting compost could be channelled into plant production, which in turn might be redirected into growing the starch to produce more biodegradable plastics.
AN OLYMPIC EFFORT - RECYCLING 76 PER CENT OF WASTE
For anyone who thinks such schemes aren't feasible, you only have to look at the recycling success of the Sydney Olympics to see that where there's a will, there's a way. More than 660 tonnes of waste was generated each day at its many venues. Of this, an impressive 76 per cent was collected and recycled. Part of this success was due to the use of biodegradable plastics used in the packaging of fast food, making the composting of food scraps an economic proposition as it eliminated the need for expensive separation of packaging waste prior to processing.
With intelligent use, these new plastics have the potential to reduce plastic litter, decrease the quantities of plastic waste going into landfills and increase the recycling of other organic components that would normally end up in landfills.